Immunotherapy and Cardiovascular Health - A Cause for Concern?
PlumX
Immunotherapy and Cardiovascular Health - A Cause for Concern?
Altmetric
Featured Article
Drobni, Zsofia D., Raza M. Alvi, Jana Taron, Amna Zafar, Sean P. Murphy, Paula K. Rambarat, Rayma C. Mosarla, et al. 2020. “Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque.” Circulation 142 (24): 2299–2311. https://doi.org/10.1161/circulationaha.120.049981.
Introduction
On May 1st, 2023 the multi-institutional Society of Cutaneous Oncology (SoCO) Journal Club (JC) reviewed the Circulation article “Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque”.1 Participants included clinicians and investigators from Massachusetts General Hospital, Mass Eye and Ear, the National Institutes of Health, the University of Pittsburgh Medical Center and the University of Pennsylvania. Please note, the comments in this article represent the views of the authors of this Perspectives on the Science piece. It does not represent views of any other SoCO member or their affiliated institutions. In this article we provide a summary of the discussion regarding this important contribution to the literature.
Background for the Study
Immune checkpoint inhibitor therapy has revolutionized the management of skin cancer.2–4 With over 50 FDA approvals,5 immunotherapy has become the standard of care for the majority of advanced cutaneous neoplasms (Figure 1).6–8 Furthermore, recent trials have demonstrated the activity of immune checkpoint inhibitor (ICI) therapy in the peri-operative setting for high-risk resectable disease.9–16 Therefore, the cohort of skin cancer patients eligible for immunotherapy continues to expand.
While the majority of ICI-treated patients experience immune-related adverse events (irAEs), significant toxicity1 occurs in only a minority of patients receiving monotherapy anti-programmed death-1 (PD-1)/programmed cell death ligand-1 (PD-L1).17 In the setting of unresectable or metastatic disease, incidence rates of ~15% of serious irAEs are typically offset by the likelihood of clinical benefit. However, as immunotherapy becomes integrated into the management of earlier stages of disease, the risk-benefit ratio needs to be more closely scrutinized. Furthermore, in disease stages with a reasonably high likelihood of cure by local therapies (e.g. surgery or radiation), the potential for long-term sequelae of irAEs also need to be heavily considered.
1 Common Terminology Criteria for Adverse Events (CTCAE) grade 3-5.
The insight that perturbations of the CTLA-4/CD-80-86 and PD-1/PD-L1-2 pathways can trigger autoimmunity provided the biologic rationale for the use of immune checkpoint inhibitors in oncology.18 Massive lympoproliferation and fatal mutli-organ tissue destruction, including myocarditis and pancreatitis was observed in a seminal discovery using a CTLA-4 knockout mouse model.19 Thus, it has been known for nearly thirty years that immune checkpoints are critical in governing tolerance mechanisms involving auto-reactivity against the myocardium. More recent attention, however, has been placed on the role of these regulatory pathways in atherosclerosis and the potential to trigger cardioembolic events.20 Years of pre-clinical and clinical research have established chronic inflammation as critical in the pathogenesis of atherosclerotic plaque development.21 Innate immune cells, such as M1 marcrophages, can promote plaque formation via the secretion of pro-inflammatory proteins, including interleukein (IL)-1𝞫, IL-6 and tumor necrosis factor (TNF)-𝜶.22 In addition, the role of the adaptive immune system in atherosclerosis has been demonstrated in pre-clinical models of immune checkpoints. In genetic models, PD-L1/2 knockout mice have increased plaque burden associated with higher levels of CD4+ and CD8+ T-cells.23 Similarly, over-expression of CTLA-4 in T cells in mice has been associated with decreased plaque burden and reduced CD4+ T-cell presence in atherosclerotic lesions.24 In a hypercholesterolemic mouse model, administration of soluble CTLA-4 Ig diminished CD4+ T cell activation and plaque development in ApoE3*Leiden mice.25 These data provide mechanistic rationale that immune checkpoint pathways critical in attenuating the anti-tumor response are also important negative regulators of atherosclerosis. Therefore, they may have clinical relevance to patients receiving ICI-based immunotherapy.
The role of ICI in cardiovascular disease is particularly relevant in aging populations, such as those with non-melanoma skin cancer (NMSC). The average age of diagnosis of NMSC is approximately 70.26 Indeed, the vast majority (~80%) of patients diagnosed with a keratinocyte carcinoma2 are in their sixth, seventh or eighth decade of life.26 In a recent study of neoadjuvant anti-PD1 in high-risk resectable cutaneous squamous cell carcinoma (CSCC), 5% of patients (4/79) experienced a fatal event.13 Although none of the events were deemed likely related to study drug, three were cardiovascular in nature. A 93-year-old woman with a history of heart failure and paroxysmal atrial fibrillation died secondary to exacerbation of underlying congestive heart failure following two doses of cemiplimab. Second, an 85-year-old male with a history of peripheral vascular disease and coronary artery disease expired from a myocardial infarction after three doses of cemiplimab. Third, a 73-year-old male with diabetes, heart failure and atrial fibrillation died following a single dose of cemiplimab after suffering a myocardial infarction. Due to their underlying comorbidities, only the case of heart failure was adjudicated as possibly related to the study drug.3 Both myocardial infarction cases were considered unrelated to cemiplimab by the study investigators.
2 Keratinocyte carcinoma (KCC) are comprised of basal cell carcinoma and squamous cell carcinoma
3 Clinical trial investigators assign attribution to an CTCAE adverse event (AE) using the following attribution categories: Unrelated (the AE is NOT related to the intervention); Unlikely (the AE is doubtfully related); Possible (the AE may be related); Probable (the AE is likely related); Definite (the AE is clearly related). Designations of “unrelated” and “unlikely” define the relationship is unrelated to the investigational agent. “Possible”, “probable”, and “definite” connote the AE is related.
Prior to the study by Drobni et al.,1 limited data existed regarding the association of ICI and cardiovascular events. In a small study of 92 non-small cell lung cancer (NSCLC) subjects, no increase in vascular events were seen with ICI compared to cytotoxic chemotherapy.27 Similarly, in a single-institution study of 135 subjects with NSCLC, no association was linked between ICI and heart failure exacerbation, non-fatal MI or cardiovascular death.28 On the contrary, in a large exploratory observational study of 59 trials submitted to the US Food and Drug administration, a 35% increase in coronary ischemia was found in ICI-treated patients compared with those treated with cytotoxic chemotherapy.29 Therefore, to gain further insights on the potential association between ICIs and cardiovascular events, Drobni et al. conducted a large single-institution observational study at Massachusetts General Hospital.
Study Design
The primary outcome measure of the study was the development of atherosclerotic cardiovascular events, a composite outcome of myocardial infarction (MI), coronary revascularization (CR) and ischemic stroke. The investigators used both a case-control study (n = 5684) and a case-crossover design (n = 2842) to evaluate this outcome. In addition, an imaging substudy of 40 patients with melanoma was conducted to evaluate atherosclerotic plaque progression upon starting ICI therapy.
Study Results
The matched case-control study was assessed using both a univariable Cox proportional hazard model, as well as two multivariable models. In the univariable model, ICI treatment was associated with a greater than 4-fold risk of the composite adverse cardiovascular outcome (Hazard Ratio (HR) 4.68, unadjusted p-value of <0.001). Individually, all three outcomes (MI, CR or stroke) were also increased in patients treated with ICI (HR 7.16, HR 2.98, HR 4.59, respectively). In a model of known cardiovascular risk factors, ICI treatment was associated with a 3-fold increase in adverse cardiovascular outcomes. Similarly, in a forward-selection model of potential confounders, ICI use was found to have a HR of 4.5 (unadjusted p-value of <0.0001) for the composite outcome.
In the case-crossover study of 2842 patients that received ICI therapy, 139 cardiovascular events occurred in 119 patients in the two years following treatment compared with 78 events in 66 patients in the 2 year period prior to starting therapy (incidence rate ratio 1.8 [95% CI 1.4-2.4, unadjusted p-value of <0.001]).
The rate of total plaque volume progression was noted to be 6.7% per year in patients following initiation of immunotherapy. This was compared to a rate of progression of only 2.1% per year in those same patients prior to ICI (unadjusted p-value of 0.17). In a subset analysis, patients treated with a statin or corticosteroid had decreased plaque progression compared to those not on those agents.
Discussion
Immunotherapy has changed the therapeutic landscape dramatically for patients with skin cancer. Given the potential for substantial clinical benefit, their use has been expanded from locally advanced and metastatic disease to also include those with high-risk resectable disease. Consequently, heightened attention is being paid to the potential risks associated with these agents. The observational study by Drobni et al. provides an important contribution in which the totality of the evidence supports an association between immune checkpoint inhibitor therapy and adverse cardiovascular events.
Strengths of the study include the use of a large retrospective data set and a multi-modal analysis that incorporated a matched case-control cohort, case-crossover design and imaging substudy. The matched cohort and case-crossover data support an interpretation that ICI therapy may indeed be associated with myocardial infarction, coronary revascularization and stroke. The imaging substudy provides further biological rationale that accelerated atherosclerotic plaque development following ICI administration may contribute to these events.
Threats to internal and external validity4 exist, to some degree, in every scientific inquiry. Despite its strengths, this study is limited by several factors. Analytic observational studies, such as the one by Drobni et al., utilize null hypothesis significance testing (NHST) to provide probabilistic statements about its findings. NHST is designed to test hypotheses (prediction) and not, in contrast, for generating hypotheses (postdiction).30 Protocol pre-registration is an important strategy to clarify prediction from postdiction. In the current study, the lack of a pre-registered protocol limits our ability to distinguish which hypotheses were generated apriori from those tested after the results were known. For example, it is not clear from the materials and methods why the authors choose to evaluate only statins and corticosteroids as potential mitigating factors for ICI-related cardiovascular outcomes. A reader could posit, understandably, that a variety of medications were actually analyzed but only the statin and corticosteroid data proved compelling enough for inclusion. Erring on the side of caution, and interpreting these results as postdiction, minimizes the chance of apophenia.5 Similarly, the authors do not explain their approach to multiple-hypothesis testing and family-wise error rate control. While many of the effect estimates are large and the resulting p-values sufficiently low to withstand error inflation, there are several analyses that are presented as “statistically significant” that would almost certainly exceed most accepted NHST thresholds upon alpha correction. These limitations threaten the internal validity of the study’s conclusions.
4 Internal validity is defined as the extent to which the results of a investigation represent the truth in the study population and are, therefore, not due to systematic or methodologic errors inherent in the experimental system. External validity refers to the extent to which the results of an investigation are generalizable to the overall population, outside of the study.
5 Apophenia is the propensity to perceive meaningful patterns in unrelated phenomena
Control selection is often the most complex and controversial aspect of a case-control study. In particular, inferences drawn from this study design are dependent on the resulting comparability of the cases and controls. Ideally controls should be similar to cases in all respects other than having the key study variable (e.g., exposure to ICI). Due to inherent challenges in this investigation, incorporating a perfect control group is not possible. ICI is the standard of care for many stages of cancer; consequently, identifying controls that are matched by potential confounders such as decade of treatment, stage of disease and performance status is not feasible. Consequently, controls in this study were taken from a different time period from the cases. Likewise, disease stage and performance status were not used a matching criteria. In addition, there is a lack of clarity as to when the risk period began in the control group. These study elements weaken the inference that ICI therapy is associated cardiovascular events.
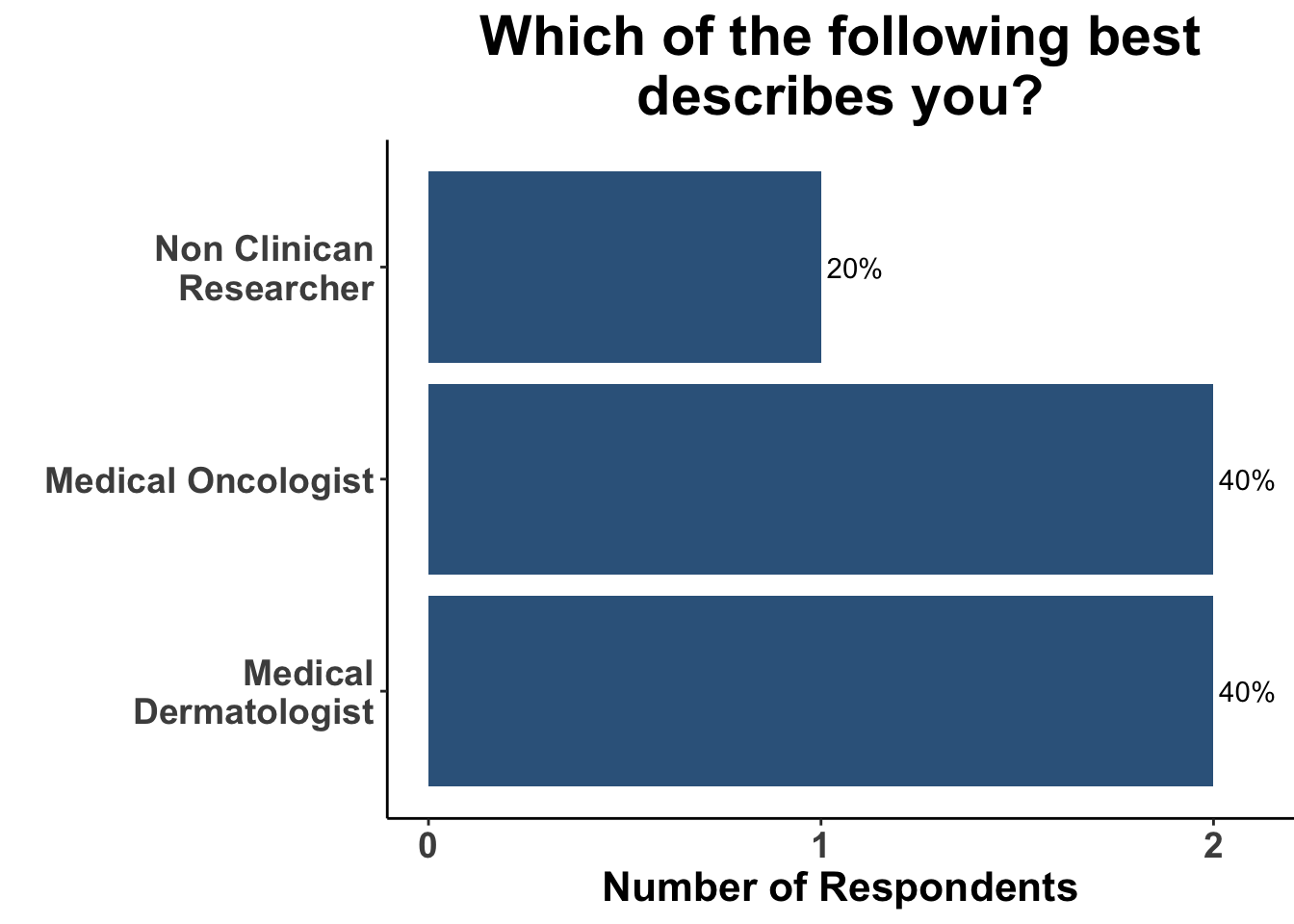
Despite those limitations, the experienced, multi-disciplinary SoCO group present on May 1st (Figure 2 and 3) acknowledged the importance of these data to their practice. The majority of the clinicians reported having had a patient develop a myocardial infarction or stroke while on treatment with an ICI (Figure 4).
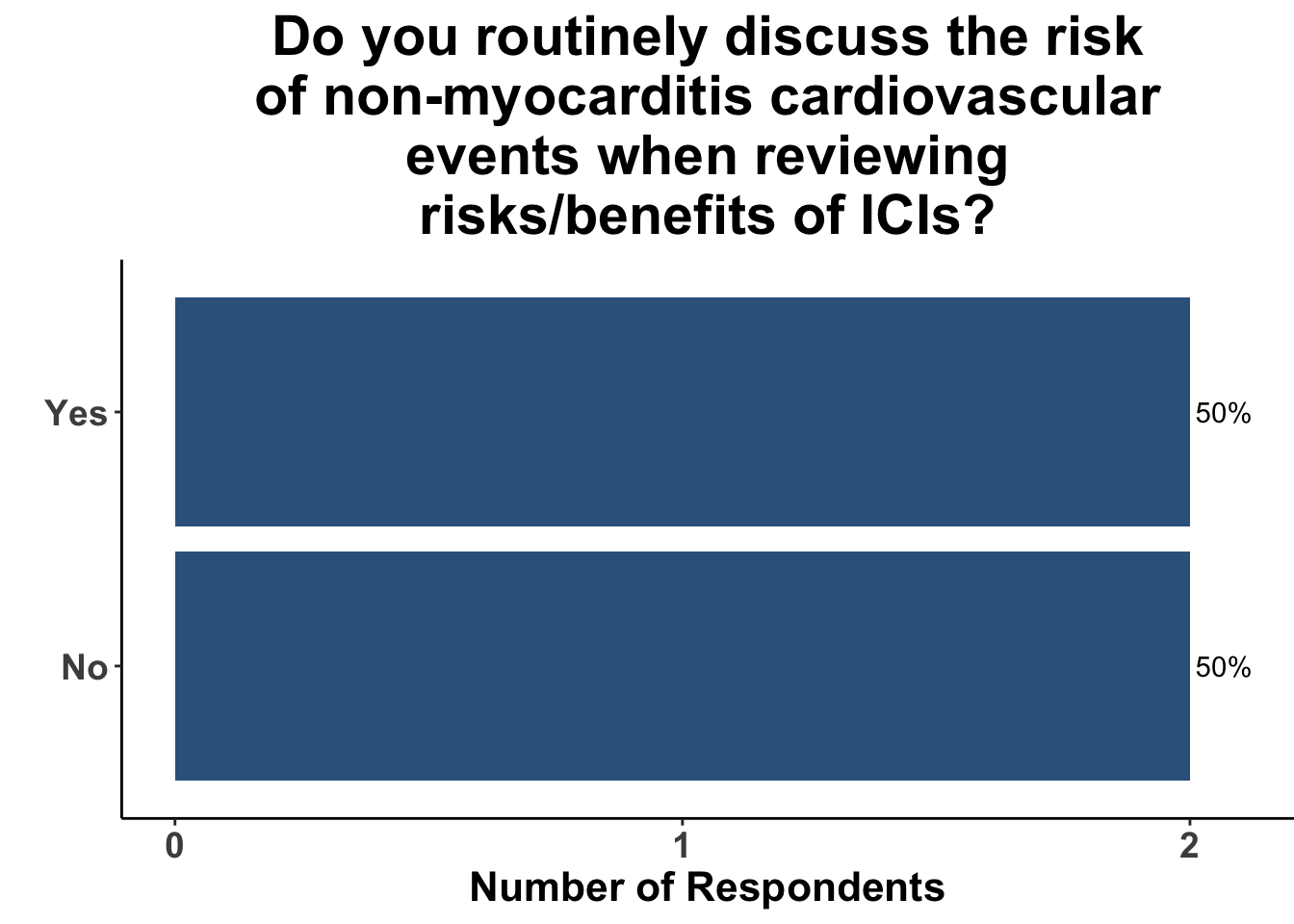
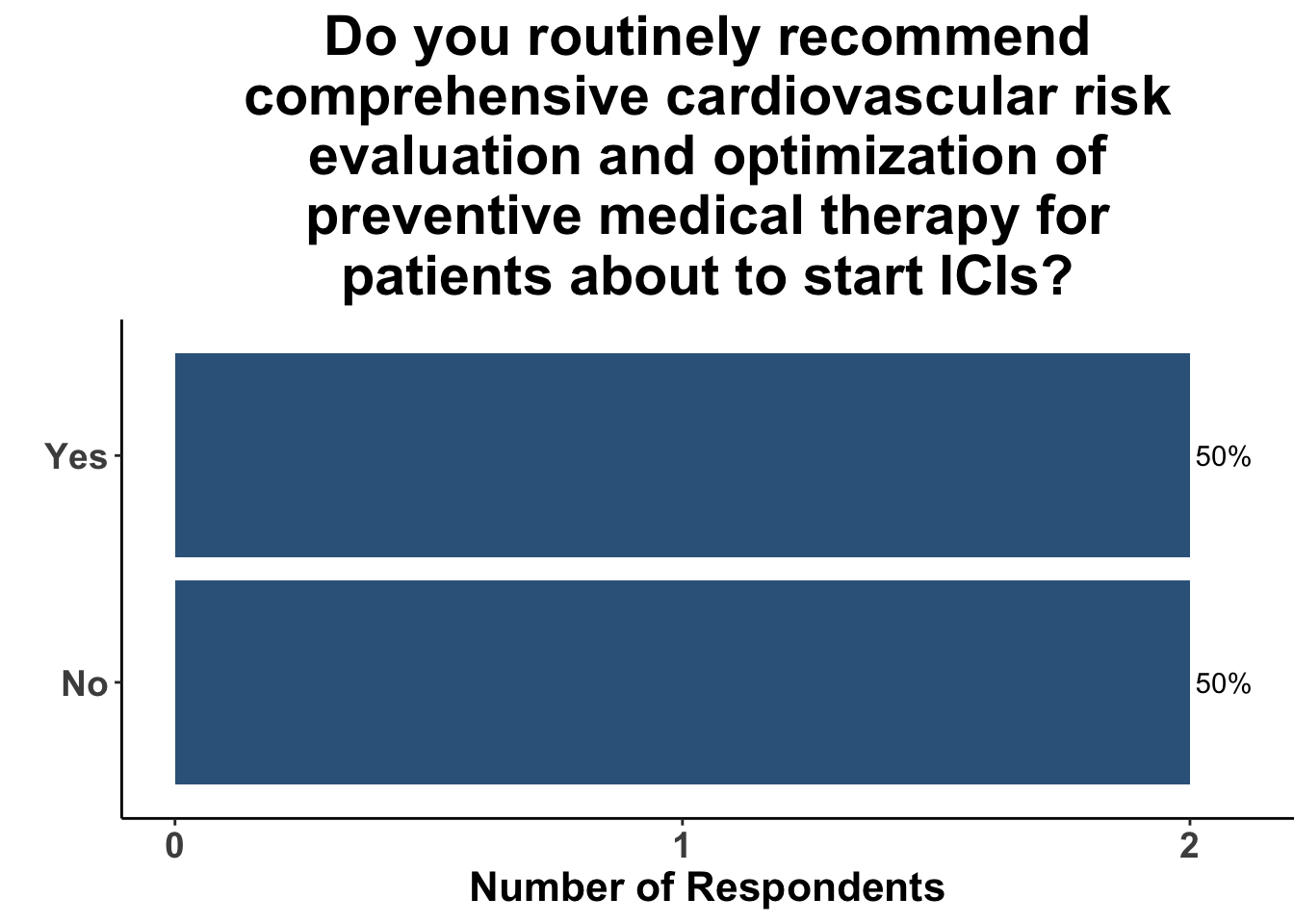
Those familiar with the study were routinely counseling patients regarding the risks of cardiovascular events (Figure 5) and recommending patients to undergo cardiovascular evaluation prior to starting therapy (Figure 6).
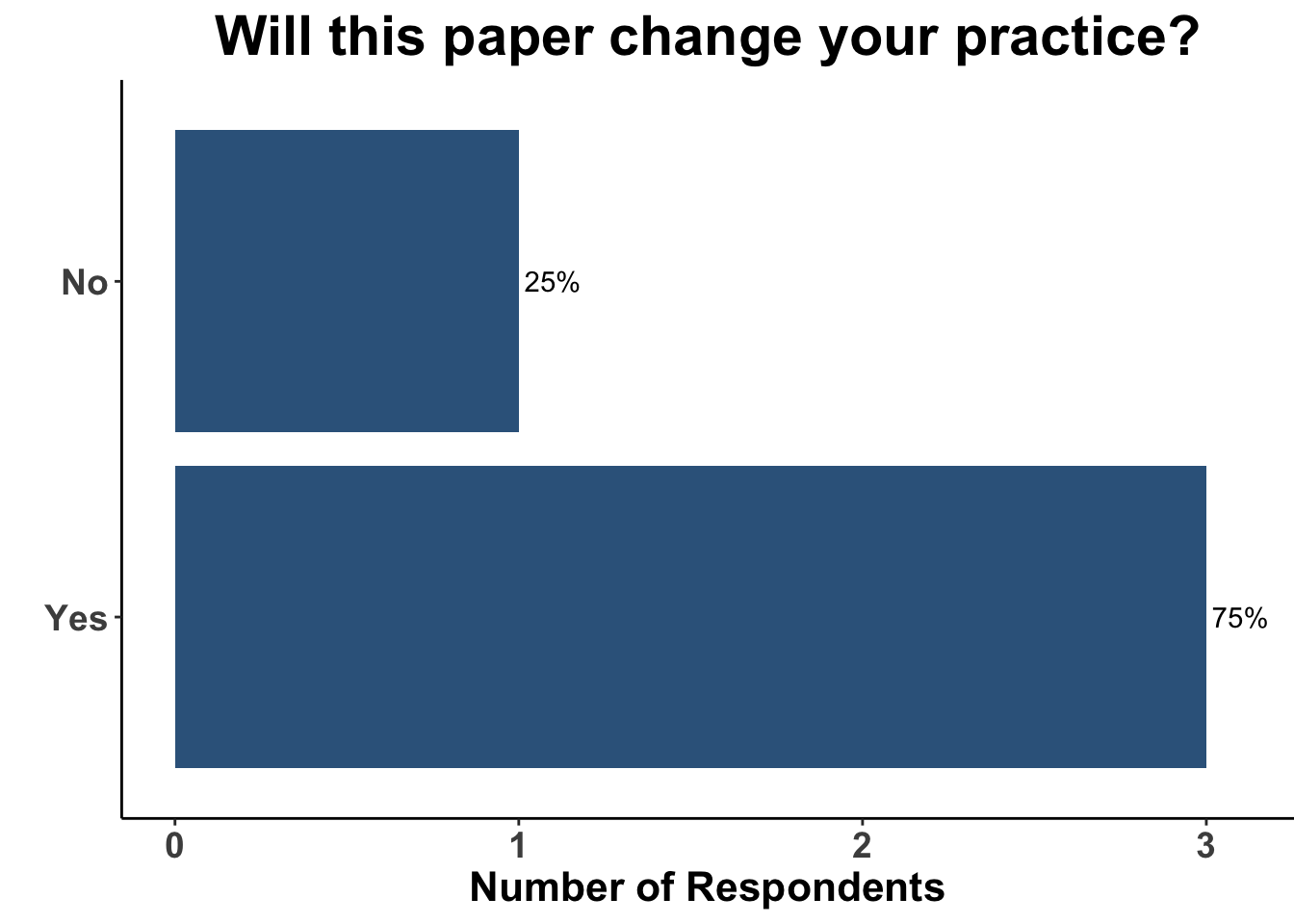
Furthermore, 75% of respondents reported that the study by Drobni et al. would change their practice (Figure 7), either by more specifically counseling patients on the potential association of immunotherapy and atherosclerosis, or by adjudicating the potential relatedness of ICI with cardiovascular events in clinical trials (Figure 8). This latter stands in contrast to the study by Gross et al.,13 in which both of the cases of fatal MI were deemed not related to ICI.
Despite the increased awareness of the possible connection between ICI and MI or stroke, it remains unclear how to effectively mitigate these potential adverse events. The findings that statin or corticosteroid use may modify the ICI-related atherosclerotic plaque progression are most appropriately considered hypothesis generating. As a result, many important unanswered questions remain about this connection. For example, the effect of timing and dose of either agent were not studied. Both statins and corticosteroids have the potential for their own adverse events. While the impact of corticosteroids on the anti-tumor response remains incompletely understood, available data suggests that both timing of administration and total drug exposure are important factors in potentially reducing the efficacy of immunotherapy.31 In regards to statin therapy, in a recent study of 3000 ICI-treated subjects, statin use was associated with a 2-fold higher risk of myopathy.32 Thus, appropriately-sized, prospective clinical trials are needed to evaluate methods to potentially attenuate the likely association of ICI therapy and cardiovascular events.
In addition to better understanding potential mitigating factors, several other questions remain. For example, is the association between ICI threapy and cardiovascular events exposure-dependent? Does dual-immune checkpoint blockade (e.g anti-CTLA-4/PD-1 or anti-LAG3/PD-1) confer a higher risk of adverse cardiovascular outcomes than monotherapy ICI? How long after exposure to ICI therapy does the increased risk of MI or stroke persist? These questions are critical as we try and determine the balance of risks and benefits of peri-operative immunotherapy. Most neoadjuvant regimens for skin cancer incorporate between one to four doses of pre-operative immune checkpoint blockade. In the study by Drobni et al. single-agent anti-PD-1 was used in 75% of the cases and the median number of cycles of ICI administered was five. Thus, we cannot determine from this data set whether there is a exposure- or regimen-dependency to this association. Similarly, there is a lack of clarity on the length of the association of ICI with MI or stroke following treatment induction. The kaplan-meier curves from the study suggest that the effect continues for several years, but this question was not specifically addressed by this investigation. If only a single dose of ICI therapy confers a prolonged risk of MI or stroke for patients, the enthusiasm for pre-operative anti-PD1 may be lessened for patients with earlier stage skin cancer.
Conclusions
Immune checkpoint inhibitor therapy has greatly changed the field of cutaneous oncology. Advances in our understanding of the immunobiology of skin cancer has provided patients and clinicians with therapeutic options in high-risk and advanced disease. We are, however, just starting to understand the role of immune checkpoints in cardiovascular biology and atherosclerotic-related events. The study by Drobni et al. provide an important contribution to the field. Additional studies will help better define the risk profile of ICI therapy and allow for more appropriate patient selection.
Materials and Methods
This Perspectives on the Science piece was published using Quarto®. The survey was conducted using REDCap®.33 The figures depicting the survey data were created using R (version 4.0.0) and the tidyverse suite of packages,34 including ggplot2.35 The image on the “Perspectives on the Science” page was created by the authors (DMM) using the rosemary package.36
Bibliography
Appendix
Citation
@article{miller2023,
author = {Miller, David M. and Ferris, Laura K. and Gupta, Sameer and
Brownell, Isaac and Shalhout, Sophia Z.},
publisher = {Society of Cutaneous Oncology},
title = {Immunotherapy and {Cardiovascular} {Health} - {A} {Cause} for
{Concern?}},
journal = {Journal of Cutaneous Oncology},
volume = {1},
number = {1},
date = {2023-05-01},
url = {https://journalofcutaneousoncology.io/perspectives/Vol_1_Issue_1/association_of_ici_with_cardiovascular_risk},
doi = {10.59449/joco.2023.05.01},
issn = {2837-1933},
langid = {en}
}