Neoadjuvant ICI for Resectable CSCC
Perspectives on the Science piece we reflect on the impact of the recently published article “Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma”.
Squamous Cell Carcinoma, Neoadjuvant, Cemiplimab
Featured Article
Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. Gross et al. NEJM. 2022 Oct 27;387(17):1557-1568. PMID: 36094839
Introduction
On October 7th, 2022, the multi-institutional Community of Cutaneous Oncology Journal Club reviewed the recently published New England Journal of Medicine (NEJM) article “Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma” (Gross et al. 2022). Participants included clinicians and investigators from Massachusetts General Hospital, Mass Eye and Ear Infirmary, Brigham and Women’s Hospital (BWH), George Washington Cancer Center, the National Institute of Health, University of Pennsylvania and Moffitt Cancer Center. Importantly, the comments in this article represent the views of the authors of this Perspectives on the Science piece after the Journal Club review. It does not represent views of any other members of the Interest Group or the affiliated institutions. Here, we provide a summary of the discussion regarding this important contribution to the literature. Of note, several participants of the Journal Club were investigators of this study and authors on the NEJM article.
Background for the Study
Although the vast majority of cutaneous squamous cell carcinomas (CSCC) are treated and cured with local destruction or surgical excision, a subset of patients have poor outcomes, including regional, nodal and distant metastases. Approximately 6% of patients with CSCC will go on to develop nodal or distant disease, resulting in a mortality of 1.5-2% (Patel et al. 2021; Thompson et al. 2016). Consequently, there is an unmet need to find alternative treatment strategies to improve outcomes in patients with high-risk CSCC.
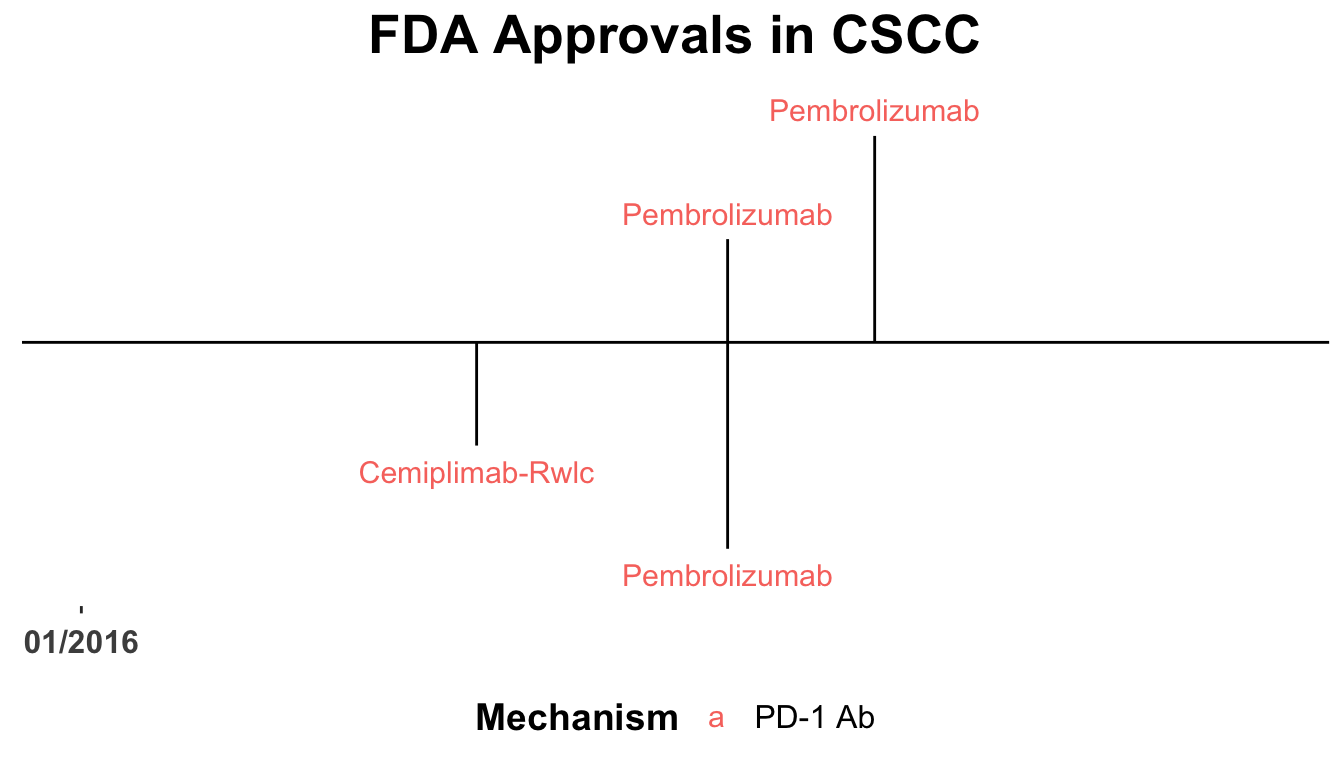
The therapeutic landscape for CSCC has rapidly evolved over the last five years (Shalhout et al. 2021, 2023; D. M. Miller et al. 2022) (Figure 1). Recently, clinical trial data has lead to the approval of immune checkpoint inhibitors for locally advanced and metastatic CSCC (Migden et al. 2018; Grob et al. 2020).
Objective response rates for advanced disease ranging from 46-51% and 35-50% in EMPOWER-CSCC-1 and KEYNOTE-629, respectively, led to the hypotheses tested by Gross et al. that pre-operative anti-PD-1 would improve pathological response rates at the time of surgical excision.
Study Design
The investigators conducted a company-sponsored, phase 2, multi-center, non-randomized study involving 79 patients with high-risk disease. Patients were eligible if they had resectable Stage II or greater disease. Of note, only Stage II patients with primary lesions >3 cm were eligible. Subjects received up to four doses of cemiplimab 350 mg every three weeks, followed by surgical resection. The primary endpoint of the study was a pathological complete response (pCR) rate. The null hypotheses was a pCR rate of 25%. Following surgery, investigators were allowed to decide on a post-operative course that included observation, adjuvant radiotherapy or continued cemiplimab.
Main Findings
Pathological complete responses were seen in 40 patients (51%), with another 10 patients exhibiting a major pathological response (defined as <10% viable tumor cells seen on the specimen). Adverse events (AEs) were seen in 87% of patients, with grade three or greater AEs occurring in 18% of patients. Four patients died during the study.
Discussion Points
The consensus amongst the Interest Group was that this study is indeed practice-changing. A few participants were already incorporating pre-operative immunecheckpoint inhibitor (ICI) therapy for some of their highest-risk patients prior the publication of these results. Other participants that were primarily using ICI therapy per the FDA indications (e.g. locally advanced or metastatic disease) stated that they would consider implementing ICI earlier in the disease journey as a result of this study. Participants, however, stressed the importance of assessing the benefit-to-risk ratio. The absolute risk of poor outcomes for various stages of disease studied in this population varies and, in many cases, remain incompletely clarified. This may be due to CSCC not being part of the Surveillance, Epidemiology, and End Results Program. Therefore prognostic estimates of local recurrence, nodal and distant metastases, as well as disease-specific death, have come largely from single-institution datasets and are limited. For example, a 2019 study by Ruiz et al. evaluated the prognosis of 459 patients at BWH (Ruiz et al. 2019). The 10-year cumulative incidence (CIN) rate of local recurrence (LR), nodal metastases (NM) and disease-specific death (DSD) for AJCC Stage II disease was 15.8, 12.2 and 7.6%, respectively in the 36 tumors evaluated. For the 119 T3 tumors (e.g. AJCC Stage III), the 10-year CIN rates for LR, NM and DSD were 19.7, 14.1 and 9.3%, respectively.
Thus the benefits of pre-operative ICI must be weighed in the setting of toxicity. The current study had 4 fatalities, an unusually high proportion, given 79 total subjects. Typical estimated rates of ICI-related mortality are 1:200. Although only one subject’s death was considered possibly related to treatment, all four died of cardiac-related events. These subjects were advanced in age (73, 82, 85 and 93 years old) and had underlying heart disease; thus, making attributions of treatment relatedness challenging. However, we must continue to be vigilant of possible treatment-related effects of ICI therapy given the advanced age of the CSCC population and emerging data that anti-PD-1 therapies may be associated with increases in cardiovascular events and atherosclerotic plaque disruption (Drobni et al. 2020).
In the wake of this impactful study, several questions remain. These include the optimal number of doses of pre-operative ICI. Comparable path CR rates were observed in a pilot study two pre-operative doses of cemiplimab in Stage II-IV CSCC (Ferrarotto et al. 2021). Twenty-two percent of the subjects in the study did not received all four doses and 11% did not undergo surgery in the protocol-specified window. Thus, identifying the optimal number of pre-operative doses of ICI therapy remains critical. In addition, understanding the clinical benefit of pre-operative ICI in some of the most at-risk patients is necessary, including those with incompetent immune systems. Furthermore, integrating pre-operative ICIs into a treatment landscape of post-operative systemic therapy, adjuvant radiation therapy or salvage treatment upon disease recurrence remains important unmet need.
Materials and Methods
This Perspectives on the Science piece was published using Quarto®. The figure depicting FDA approved therapies in CSCC was created using the skincancerRx package (D. Miller and Shalhout 2022).
References
Appendix
Disclosures
DMM reports grants and personal fees from Regeneron, grants from Kartos Therapeutics, grants from NeoImmuneTech, personal fees from Checkpoint Therapeutics, personal fees from Pfizer, personal fees from Merck Sharpe & Dome, personal fees from EMD Serono, grants from Project DataSphere, personal fees from Sanofi Genzyme, personal fees from Castle Biosciences, personal fees from Avstera, outside the submitted work. SZS reports no competing interests.
Disclaimer
This site represents our opinions only. See our full Disclaimer
Reuse
This work is licensed under a creative commons BY-NC-ND license
Citation
@article{miller2022,
author = {{Miller, David M.} and {Patel, Vishal A.} and {Cohen,
Justine V.} and {Garmen, Khalid} and {Shalhout, Sophia Z.}},
publisher = {Society of Cutaneous Oncology},
title = {Neoadjuvant {ICI} for {Resectable} {CSCC}},
journal = {Journal of Cutaneous Oncology},
volume = {1},
number = {1},
date = {2022-11-08},
url = {https://journalofcutaneousoncology.io/perspectives/Vol_1_Issue_1/neoadjuvant_ici_for_resectable_cscc/},
doi = {10.59449/joco.2022.11.08},
issn = {2837-1933},
langid = {en}
}
