Perioperative Immunotherapy for High-Risk Resectable Melanoma - A New Standard?
Neoadjuvant, Adjuvant, Immunotherapy, Melanoma
Featured Article
Patel, Sapna P., Megan Othus, Yuanbin Chen, G. Paul Wright, Kathleen J. Yost, John R. Hyngstrom, Siwen Hu-Lieskovan, et al. 2023. Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. New England Journal of Medicine 388 (9): 813–23.
Introduction
On April 7th, 2023 the multi-institutional Society of Cutaneous Oncology (SoCO) Journal Club (JC) reviewed the recently published New England Journal of Medicine article “Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma”(Patel et al. 2023). Participants included clinicians and investigators from Massachusetts General Hospital, Mass Eye and Ear, the National Institutes of Health, George Washington Cancer Center, Stanford Medical Center, Moffitt Cancer Center, University of Washington and Northwestern Medical Center. Importantly, the comments in this article represent the views of the authors of this Perspectives on the Science piece after the Journal Club. It does not represent views of any other members of the SoCO or the affiliated institutions. In this article we provide a summary of the discussion regarding this important contribution to the literature.
Background for the Study
Melanoma is a skin tumor of transformed melanocytes that is associated with high mutational burdens due to ultraviolet light exposure (Huang and Zappasodi 2022).
Melanoma incidence has increased over the past few decades with age-adjusted rates for new melanoma of the skin rising 1.2% annually over 2010-2019 (NCI 2023). Currently, melanoma comprises 5.2% of all new cancer cases in the United States (NCI 2023). The 5-year relative survival rate for those diagnosed with melanoma between 2012-2018 based on Surveillance, Epidemiology, and End Results (SEER) staging is greater than 99% for those with localized disease, 71% for regional disease, and 32% for distant disease (NCI 2023).
Melanomas are considered immunogenic, meaning antitumor immune responses are capable of killing tumor cells (Blankenstein et al. 2012; Huang and Zappasodi 2022). The immunogenicity of melanoma is thought to come from the high tumor antigen load comprised of cancer germline antigens, melanocyte differentiation antigens, overexpressed antigens, and neoantigens associated with the high mutational burden in melanomas due to UV radiation (Huang and Zappasodi 2022; Van Allen et al. 2015).
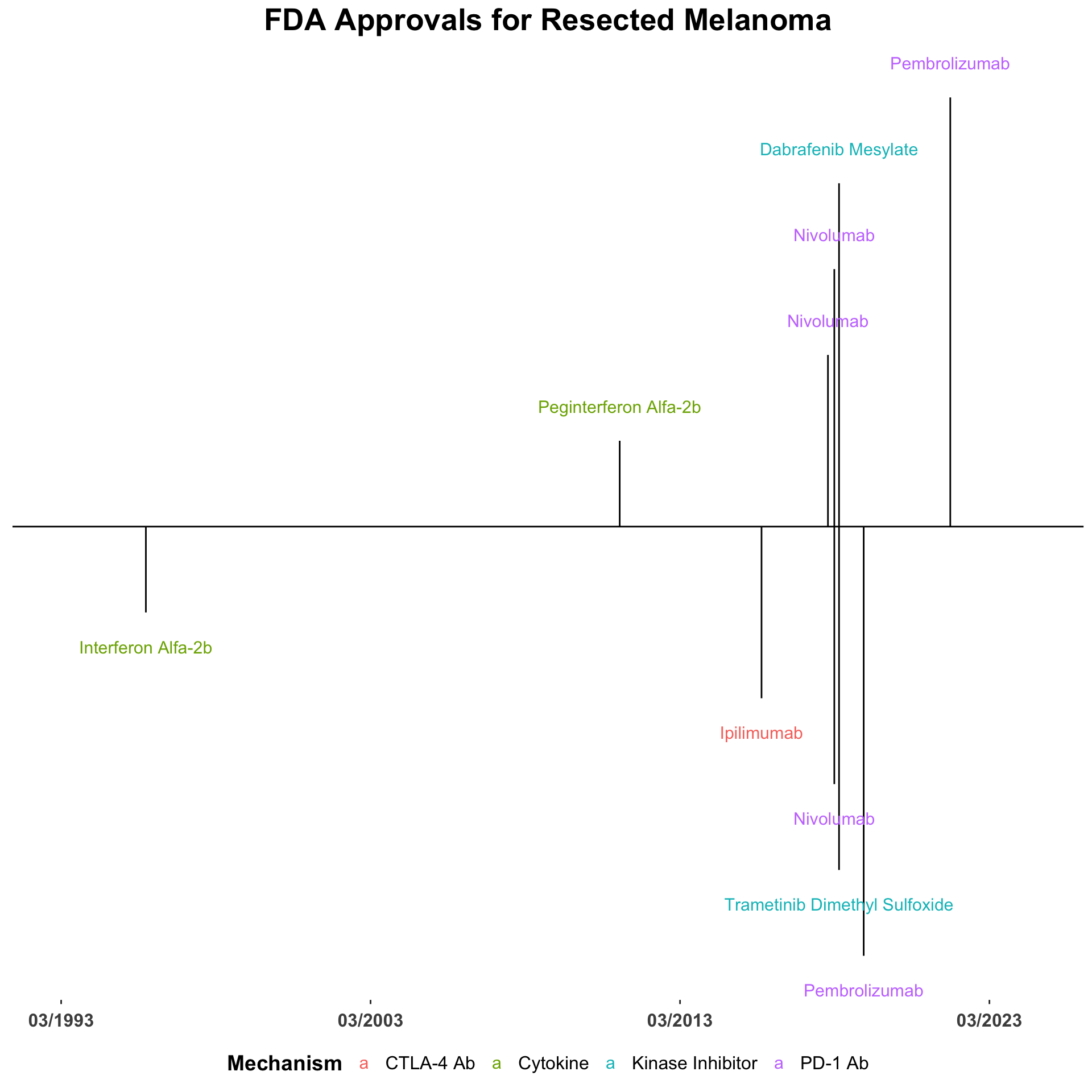
Due to the immunogenicity of melanoma, tumor progression requires a mechanism of immune escape such as the activation of immune checkpoints (Huang and Zappasodi 2022; Sharma and Allison 2015). This concept catalyzed early exploration into immunomodulators such as melanoma vaccines, the 1998 approval of recombinant IL-2 for melanoma, the 2011 approval of peginterferon-alfa-2b for melanoma, among others (Snyder, Zamarin, and Wolchok 2015; David M. Miller et al. 2022b) (Figure 1).
The current mainstay of melanoma immunotherapy blocks T cell immune checkpoints, resulting in the activation and proliferation of tumor-specific T cells (Van Allen et al. 2015). These checkpoint inhibitors include ipilimumab, an anti-Cytotoxic T Lymphocyte-Associated Protein 4 (CTLA-4) antibody, the anti-Programmed Cell Death Protein 1 (PD-1) antibodies pembrolizumab and nivolumab, and the anti-Lymphocyte Activating Gene 3 (LAG-3) antibody relatlimab (Sharma and Allison 2015; Sharpe and Pauken 2017). Checkpoint inhibitors have revolutionized the treatment of advanced stage melanoma and their use has notably improved survival in a subset of melanoma patients.
In patients with BRAF mutant melanoma, targeted therapy with BRAF-MEK inhibitors remains an important component of therapy which acts by tumor intrinsic, rather than immunomodulatory, mechanisms (Lelliott et al. 2021). Targeted therapy is associated with a high initial response rate but eventual drug resistance is a major limitation of this approach. In a recent randomized study of targeted therapy and immunotherapy, patients with BRAF mutant melanoma demonstrated improved two-year overall survival if they received immunotherapy as the first line of treatment (71.8% (95% CI, 62.5 to 79.1) vs. arm 51.5% (95% CI, 41.7 to 60.4; log-rank P = .010) (Atkins et al. 2023).
The use of adjuvant checkpoint inhibitors after complete tumor resection has been tested in numerous phase II and III trials. In 2015, the FDA expanded the approval of ipilimumab to include adjuvant therapy in stage III melanoma at high risk of recurrence following the results of the EORTC 18071 trial (Grossmann et al. 2022a; Eggermont et al. 2016). In this trial, adjuvant ipilimumab was associated with increased rate of recurrence-free survival at 5 years compared to placebo (40.8% vs. 30.3%; HR for recurrence or death, 0.76; 95% CI: 0.64 to 0.89; P<0.001) (Eggermont et al. 2016). Adjuvant ipilimumab was associated with significantly longer overall survival (65.4% vs. 54.4%; HR for death from any cause, 0.72; 95% CI: 0.58 to 0.88; P = 0.001), and distant metastasis-free survival compared to placebo (48.3% vs. 38.9%; HR for distant metastasis or death, 0.76; 95.8% CI: 0.64 to 0.92; P = 0.002) (Eggermont et al. 2016).
FDA approval for adjuvant use of anti-PD-1 antibodies followed (Figure 2). Nivolumab was FDA approved as adjuvant therapy for advanced-stage melanoma following the results of the phase III CheckMate 238 trial which demonstrated adjuvant nivolumab improved 12-month RFS (70.5%, 95% CI: 66.1-74.5 vs. 60.8%, 95% CI: 56.0-65.2) and was associated with a lower rate of serious adverse events of any grade compared to ipilimumab (17.5% vs. 40.4%) in patients with resected stage IIIB, IIIC, or IV melanoma (Weber et al. 2017).
Pembrolizumab was FDA approved as adjuvant therapy for resected stage III melanoma following the results of the placebo-controlled phase III KEYNOTE-054 trial. In this study, pembrolizumab administered every three weeks for up to one year was associated with longer 12-month recurrence-free survival compared to placebo (75.4%, 95% CI: 71.3–78.9 vs. 61.0%, 95% CI: 56.5–65.1) (Eggermont et al. 2018). In a subsequent randomized phase III trial (S1404), adjuvant pembrolizumab showed improved RFS and a more favorable toxicity profile compared to interferon alfa-2b or ipilimumab in patients with resected stage III or IV melanoma (HR for recurrence or death 0.77; 99.62% CI: 0.59-0.99; log-rank P = 0.002) (Grossmann et al. 2022b). Combined with the favorable safety profile demonstrated by PD-1 blockade over CTLA-4 blockade, these results have positioned PD-1 inhibitors as the standard of care for adjuvant melanoma treatment over ipilimumab.
Recently, trials have investigated the utility of administering neoadjuvant immune checkpoint inhibitors or targeted therapy in in high-risk resectable melanoma (Tables 1 and 2) (Blank et al. 2018; Rodabe N. Amaria et al. 2018b; Rodabe N. Amaria et al. 2018a; Rozeman et al. 2019; Long et al. 2019; Reijers et al. 2022a). The rationale for neoadjuvant immune checkpoint therapy is to give antitumor T cells time to proliferate before the bulk of the immunogenic tumor is resected (Patel et al. 2023). This strategy has been hypothesized to decrease the risk of recurrence and improve clinical outcomes and is a burgeoning field of study.
In the phase I and II OpACIN-Neo trials, neoadjuvant nivolumab plus ipilimumab decreased morbidity after tumor resection, had a favorable safety profile, and led to major pathological response rates of around 75% and high recurrence free survival in patients with macroscopic stage III melanoma (Table 1) (Blank et al. 2018; Rozeman et al. 2019; Menzies et al. 2021; Versluis et al. 2021). Despite these promising results, one concern about neoadjuvant treatments in melanoma is that they delay definitive surgical excision and thus may result in tumor progression or toxicity that prevents eventual tumor resection.
Some patients with advanced stage melanoma demonstrate near complete pathological response and favorable outcomes on neoadjuvant therapy alone, calling into question the need for subsequent tumor resection. The open-label phase II PRADO extension cohort of the OpACIN-neo trial attempted to explore outcomes in the absence of surgery in advanced-stage melanoma patients with major pathological response (mPR, less than or equal to 10% viable tumor) in their largest lymph node metastasis at baseline after neoadjuvant therapy with combination nivolumab and ipilimumab (Table 1) (Reijers et al. 2022a). In the trial, 61% of patients with stage IIIB or IIIC melanoma demonstrated mPR (Reijers et al. 2022a). At follow up, patients with mPR demonstrated 93% relapse free survival and 98% distant metastasis free survival at two-years and higher quality of life (Reijers et al. 2022a). The findings of this study further support a role for neoadjuvant immunotherapy in the treatment of advanced stage melanoma.
A phase II clinical trial demonstrated a combination regimen of neoadjuvant relatlimab and nivolumab led to favorable survival outcomes and high pathological response rates in 30 patients with resectable stage III or IV melanoma (Table 1). The combination therapy was associated with a 57% complete pathological response rate, low toxicity, and 1- and 2- year recurrence-free survival rates of 100% and 92%, respectively, for those who had any pathological response to treatment (Rodabe N. Amaria et al. 2022; David M. Miller et al. 2022a).
While neoadjuvant immunotherapy has gained momentum in the field, there are many unanswered questions that perists. This includes defining the role of subsequent melanoma excision and sentinel lymph node biopy as well the the unclear role of adjuvant therapy following a major pathologic response after neoadjuvant immunotherapy. In addition, the impact of neoadjuvant treatment on adverse events and potential for chronic toxicity are not well defined. Unexpected toxicity could could affect surgical eligibility, patient quality of life, and subsequent access to immunotherapy at the time of recurrence. Since combination immunotherapy may be associated with a higher incidence of adverse events, monotherapy approaches are especially appealing.
Study Design
In this report, Patel and colleagues conducted a phase II randomized controlled trial to assess the advantage of neoadjuvant-adjuvant pembrolizumab in increasing event-free survival in resectable stage III and IV melanoma over adjuvant anti-PD1 alone.
From February 2019 to May 2022, 313 patients from 90 sites in the United States with resectable stage IIIB, IIIC, IIID, or stage IV melanoma were enrolled in the study: 154 were randomized to the neoadjuvant-adjuvant arm, and 159 were randomized to the adjuvant-only arm. Participants in the neoadjuvant-adjuvant arm were treated with 3 doses of pre-operative pembrolizumab followed by 15 doses of post-operative pembrolizumab. Participants in the adjuvant-only arm received 18 doses of post-operative pembrolizumab. The primary endpoint was event-free survival (EFS) in the intention-to-treat population from the date of randomization. Events were defined as disease progression that prevented surgery, toxic effects of treatment that prevented surgery, inability to resect all gross disease, disease progression, surgical complications, toxic effects that prevented initiation of adjuvant therapy within 84 days of surgical excision, and death from any cause.
Study Results
After the completion of neoadjuvant therapy, 9 of 142 participants (6%) had a complete imaging-based response, 58 of 142 participants (41%) had a partial imaging-based response, and 28 of 132 participants (21%) had a complete pathological response. Across both arms, 105 events occurred: 38 in the neoadjuvant-adjuvant group and 67 in the adjuvant-only group. During a median follow up of 14.7 months, the event-free survival was significantly longer in the neoadjuvant-adjuvant group compared to the adjuvant-only group (72%, 95% CI: 64-80 vs. 49%, 95% CI: 41-59).
Less than 10% of patients in the neoadjuvant-adjuvant arm experienced toxic effects or disease recurrence that prevented surgery. No new toxic effects of pembrolizumab were detected and no deaths were attributed by investigators to pembrolizumab treatment.
Discussion
The management of resected melanoma has changed significantly over the last decade. Adjuvant indications for ipilimumab, nivolumab, pembrolizumab and dabrafenib/trametinb have given clinicians a variety of post-operative options. More recently, data demonstrating the utility of pre-operative systemic therapy have provided the rationale for additional, albeit off-label, approaches. While providers now have an assortment of choices, selection of the optimal strategy is challenging. Most studies do not directly compare emerging strategies and FDA approvals and clinical guidelines often lag behind published studies by months, sometimes years.
Underscoring this rapidly evolving therapeutic landscape, when queried as to what initial strategy they would have recommended six months ago for a patient with Stage IIIC melanoma, only a fraction of the experienced attendees of the April 7th SoCO journal club (Figures 3-6) selected pre-operative systemic therapy (Figure 6). The majority (88%), in contrast, were recommending surgery and adjuvant anti-PD1. None of the attendees would have recommended surgery followed by active surveillance for a patient presenting with clinically-detected regional metastases.
Despite the lack of an FDA-approval for pre-operative systemic therapy, recent evidence supporting the utility of neoadjuvant strategies has begun to influence medical decision making. For example, nearly one-half of SoCO JC respondents replied that they had previously recommended neoadjuvant immune checkpoint inhibitor (ICI) therapy for high-risk resectable melanoma (46%) (Figure 7).
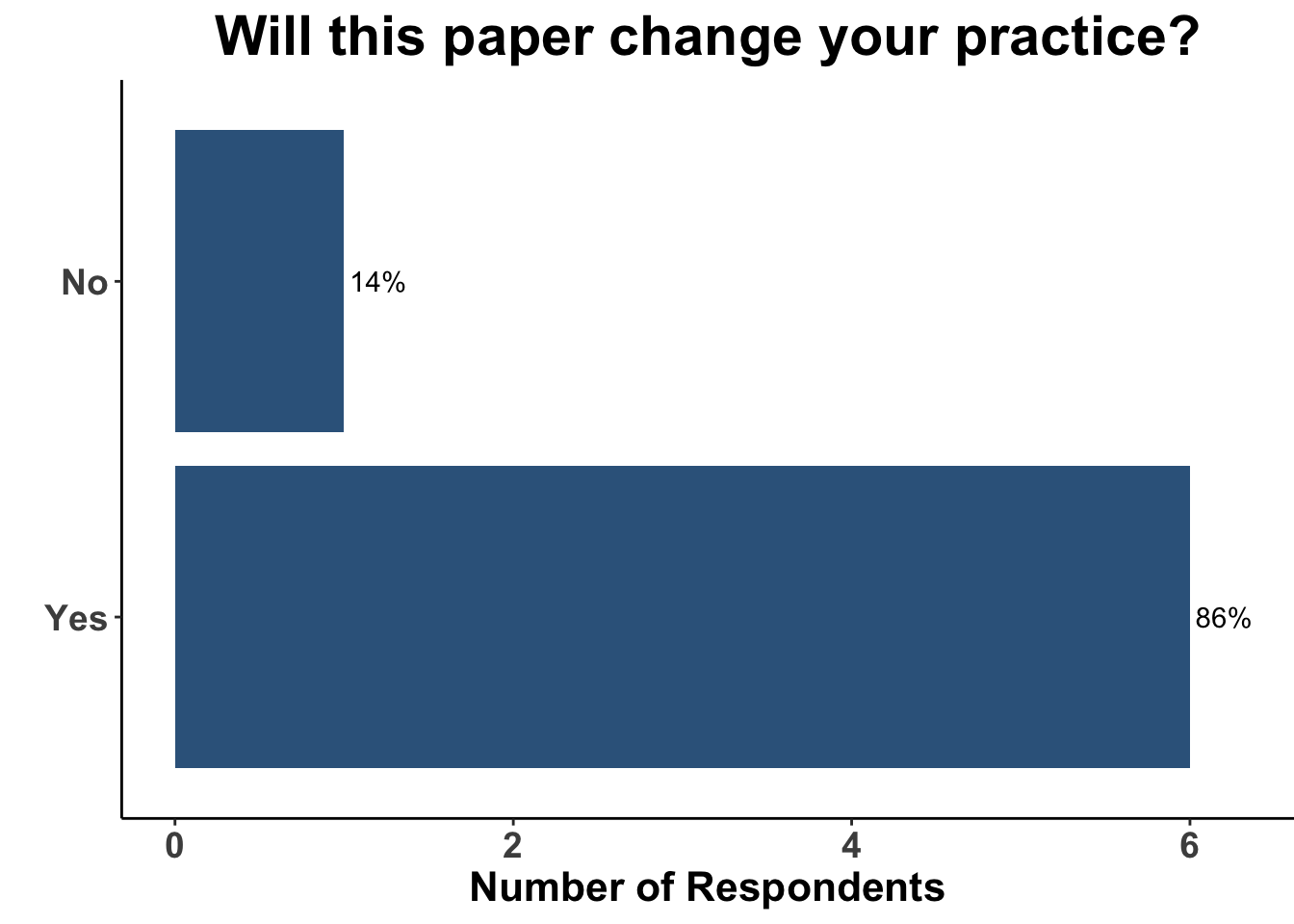
Given the strength of the data from SWOG S1801, the use of pre-operative ICI will likely only increase. Indeed, 86% of SoCO melanoma clinicians present on April 7th responded that this study would in fact change their practice (Figure 8).
Indicative of this impact, the majority of practioners (83%) now stated that they would incorporate neoadjuvant immunotherapy as part of the initial management plan for a patient with T3bN1bM0 disease (Figure 9). In contrast, only 12% responded they would have done so six months ago (Figure 6). Provided with options of monotherapy anti-PD1 or dual immune checkpoint blockade, 57% of clinical attendees replied they would utilize monotherapy neoadjuvant anti-PD1, while one would recommend combination neoadjuvant nivolumab/relatlimab. None replied they would recommend neoadjuvant ipilimumab/nivolumab for this type of case today (Figure 9).
Will peri-operative anti-PD1 become the new standard of care? It is unclear. Despite the impressive results reported by Patel et al, questions and concerns remain. Although the endpoint of EFS is appropriate and informative, aggregate endpoints have inherent challenges. Collapsing parameters such as failure to receive surgery and relapse with death, for example, conflates the relative importance of arguably disparate outcomes. Indeed, as a result of the study not having shown a difference in overall survival (OS was an exploratory endpoint and there was no obvious early signal of significant separation between the curves), several SoCO participants argued that post-operative-only anti-PD1 remains a very reasonable option for patients with high-risk resectable disease. . In addition, the median follow-up in this report was less than 15 months and historic data in melanoma has shown that survival advantage may be limited once longer follow-up is available. Thus, it will be important to continue to follow the patients in Patel et al. out to five, and even ten, tears for survival.
In addition, there were concerns about the imbalance in the number of patients that were unable to undergo surgery. Specifically, 14 subjects in the neoadjuvant-adjuvant group failed to undergo surgery due to either toxicity (n = 1), disease progression (n = 12) or medical co-morbidities (n = 1). This is compared to just 1 subject in the adjuvant-only group, who did not receive surgery for a non-COVID scheduling issue. Ultimately, it is unknown if those patients who progressed prior to surgery would have had meaningful clinical benefit from surgery or if their outcomes were predominantly determined by underlying aggressive biology.
Surgical excision of resectable melanoma remains the standard of care. Thus, pre-operative strategies that delay and possibly preclude surgery is a legitimate concern. Importantly, the disparity in cases able to proceed to surgery raises the question of how many pre-operative doses of immunotherapy are needed to optimally prime the immune system. Would fewer doses of upfront therapy reduce the number of surgery ineligible patients, while simultaneously preserving efficacy? Although cross-disease and cross-trial comparisons are limited by several important caveats, important insights may be elucidated. For example, in cutaneous squamous cell carcinoma (CSCC), extra doses of neoadjuvant anti-PD1 therapy did not appear to yield higher pathological response rates, though it may be associated with more failures to undergo surgery. In a small study of 20 patients with high-risk, resectable CSCC, two doses of anti-PD1 resulted in mPR in 70% of subjects (Ferrarotto et al. 2021). In that study, all 20 patients underwent surgical excision in the study window. In contrast, 11% of patients failed to have surgery during the study window in a larger follow-up trial of 79 subjects designed to receive four doses of neoadjuvant cemiplimab (Gross et al. 2022). In this study, 63% of patients that received up to four doses of cemiplimab had a mPR. Thus, future studies investigating the minimal number of pre-operative doses necessary for clinical benefit are needed in both melanoma and CSCC. Furthermore, sentinel lymph node biopsy is a critical part of the management for patients with intermediate thickness melanoma and impact staging. Adoption of neoadjuvant immunotherapy needs to consider when and how sentinel lymph node sampling might occur or, indeed, whether it could be eliminated.
Other unanswered questions include the role of adjuvant immunotherapy for patients who achieve a mPR following neoadjuvant immunotherapy. In the PRADO trial, the outcome for patients that had a mPR following neoadjuvant ipilimumab/nivolumab was excellent (Reijers et al. 2022b). The estimated 24-month RFS in the absence of any post-operative systemic therapy was 93%. Thus, further trials evaluating the outcomes of patients with mPR after receiving monotherapy anti-PD1 are necessary.
Conclusion
Whether or not peri-operative immunotherapy becomes the standard of care for high-risk resectable melanoma remains to be seen. The results from the SWOG S1801 trial, however, do clearly reinforce the rationale for future trials that incorporate pre-operative systemic therapy, followed by tailored approaches to post-operative management based on pathological assessment of resected specimens. Finally, there was general enthusiasm for considering the results of S1801 in melanoma for other cutaneous malignancies. Further focus on neoadjuvant immunotherapy for non-melanoma skin cancer is anticipated to be a major area for future clinical investigation.
Materials and Methods
This Perspectives on the Science piece was published using Quarto®. The survey was conducted using REDCap® (Harris et al. 2009). The figures depicting the survey data were created using R (version 4.0.0) and the tidyverse suite of packages (Wickham et al. 2019), including ggplot2 (Wickham 2016). The image on the “Perspectives on the Science” page was created by the authors (DMM) using R and ggplot2 (Wickham 2016). The figures depicting FDA approved therapies in skin cancer were created using the skincancerRx package (D. Miller and Shalhout 2022). The image on the “Perspectives on the Science” page was created by the authors (DMM) using the rosemary package.(Navarro 2023)
Bibliography
Appendix
Citation
@article{vilasi2023,
author = {Vilasi, Serena and Miller, David M. and Kaufman, Howard L.
and Emerick, Kevin S. and Shalhout, Sophia Z. and Kim, Emily Y and
Patel, Vishal A. and Garmen, Khalid and Gupta, Sameer and Brownell,
Isaac},
publisher = {Society of Cutaneous Oncology},
title = {Perioperative {Immunotherapy} for {High-Risk} {Resectable}
{Melanoma} - {A} {New} {Standard?}},
journal = {Journal of Cutaneous Oncology},
volume = {1},
number = {1},
date = {2023-04-15},
url = {https://journalofcutaneousoncology.io/perspectives/Vol_1_Issue_1/perioperative_immunotherapy_for_high_risk_melanoma/},
doi = {10.59449/joco.2023.04.15},
issn = {2837-1933},
langid = {en}
}