Tumor-Infiltrating Lymphocyte Therapy for Advanced Melanoma: Ready for Prime Time?
Melanoma, Tumor-Infiltrating Lymphocytes
Featured Article
Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. Rohaan et al. NEJM. 2022 Dec 8;387(23):2113-2125.
Introduction
On January 9th, 2023, the multi-institutional Community of Cutaneous Oncology Journal Club reviewed the recently published NEJM article “Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma”(Rohaan et al. 2022). Participants included clinicians and investigators from Massachusetts General Hospital, Mass Eye and Ear, Brigham and Women’s Hospital, the National Institutes of Health, George Washington Medical Center, the University of Pittsburgh Medical Center and the Northwestern Feinberg School of Medicine. Importantly, the comments in this article represent the views of the authors of this Perspectives on the Science piece after the Journal Club. It does not represent views of any other members of the Interest Group or the affiliated institutions. In this article we provide a summary of the discussion regarding this important contribution to the literature.
Background for the Study
Treatment options for melanoma have advanced greatly in the last several years and continue to evolve (Figure 1). Landmark studies such as KEYNOTE-001/006 and CheckMate-066 have led to single-agent immunotherapy with anti-PD-1 immune checkpoint inhibitors becoming a first-line treatment options for advanced, unresectable melanoma (Figure 2)(Hamid et al. 2019; Robert et al. 2015; Robert, Ribas, et al. 2019; Schachter et al. 2017; Petrella et al. 2017). Subsequent studies such as CheckMate-067 and RELATIVITY-047 have positioned dual immune checkpoint blockade (ICB) with aPD-1/aCTLA-4 and aPD-1/aLAG-3 monoclonal antibodies as additional options in the front-line setting for appropriate patients (Wolchok et al. 2017; Tawbi et al. 2022; Long et al. 2022). In addition combined BRAF and MEK inhibition is an option for melanomas with BRAF mutations (Robert, Grob, et al. 2019; Dummer et al. 2018).
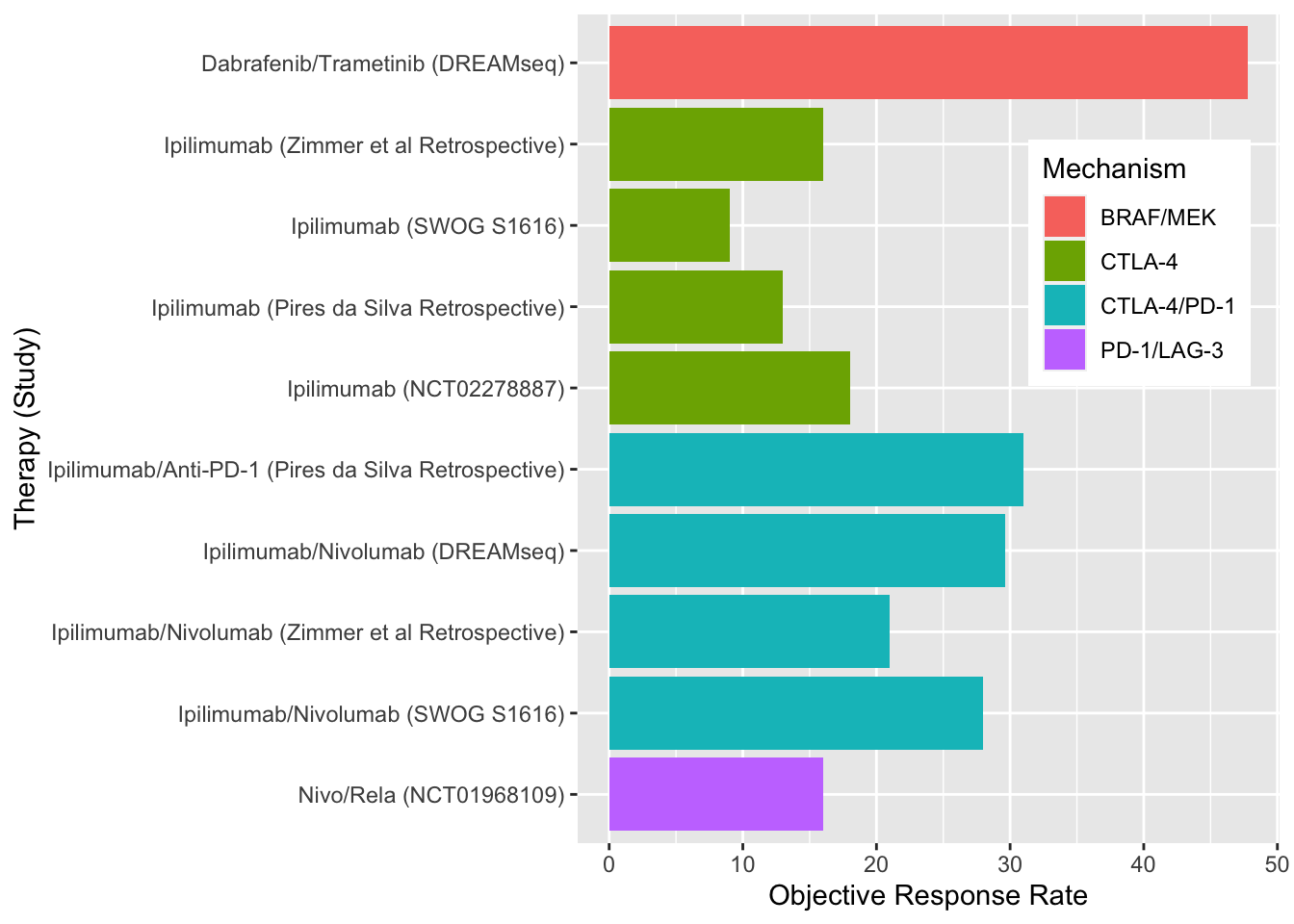
Despite these treatment measures, primary and acquired resistance is common for all front-line therapeutic options. Unfortunately, outcomes with ICI and targeted therapies in the second-line setting have been disappointing (Figure 3). (Atkins et al. 2023; Pires da Silva et al. 2021) Subsequently, the 5 year mortality rate in patients with stage IV disease is 50%.
Adoptive cell therapy with tumor-infiltrating lymphocytes (TILs) has shown clinical utility in melanoma since the 1990s (S. A. Rosenberg et al. 1994). In this process, tumor-resident T cells from a patient’s tumor are extracted and expanded ex-vivo. The patient is administered preparative lymphodepleting chemotherapy, often with cyclophosphamide and fludarabine, before the T-cells are infused back into the patient with interleukin-2 to further enhance in vivo expansion of cells and boost the antitumor response. While results have varied, multiple phase I and II trials have shown response rates around 50% (Berg et al. 2020; M. E. Dudley et al. 2008; Steven A. Rosenberg et al. 2011; Besser et al. 2010; Ellebaek et al. 2012; Andersen et al. 2016; M. Dudley et al. 2022; Pilon-Thomas et al. 2012). Several of these studies are summarized in Table 1. C-144-01, a recently reported study of LN-144 TIL therapy in patients who failed anti-PD-1 immunotherapy reported an objective response rate of 31.4% in a pooled analysis of consecutive cohorts, highlighting potential utility of TIL therapy for patients who have had disease progression on first-line options (Sarnaik et al. 2021).
Study Design
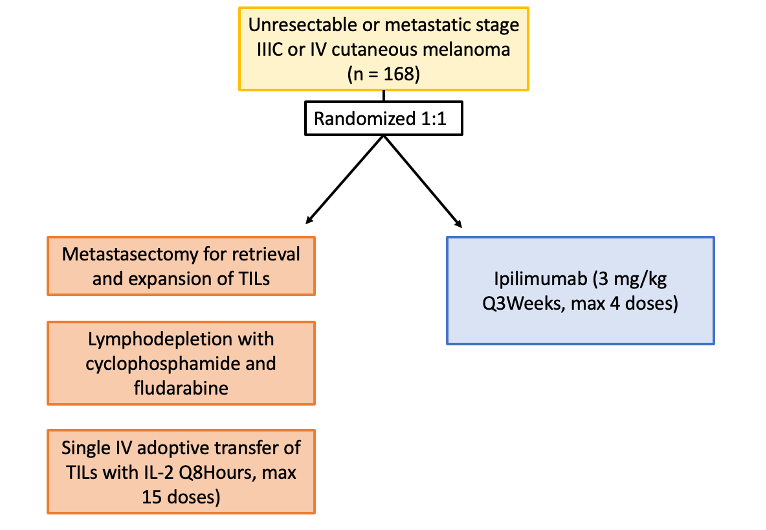
The study by Rohaan et al. was a phase III, multicenter, open-label trial including Dutch patients age 18-75 with unresectable stage IIIC or IV melanoma. Patients were randomized in a 1:1 ratio to receive TIL or anti-cytotoxic T-lymphocyte antigen 4 (CTLA4) therapy (ipilimumab) (Figure 4). Patients assigned to the TIL group underwent a metastasectomy for retrieval and expansion of TILs, then received nonmyeloablative, lymphodepleting chemotherapy with cyclophosphamide and fludarabine. Single intravenous adoptive transfer of TILs was followed by high-dose interleukin-2 every 8 hours, for a maximum of 15 doses. Patients in the ipilimumab group received 3 mg per kilogram of body weight every 3 weeks, for up to 4 doses. The primary end point was progression-free survival, defined as the time from randomization to first disease progression or death. Secondary endpoints included progression-free survival (PFS), objective response, complete response, overall survival, health-related quality of life, and safety.
Main Findings
168 patients (86% with disease refractory to anti–programmed death 1 treatment) were assigned to receive TILs (84 patients) or ipilimumab (84 patients). Median PFS in the TIL group was 7.2 months, significantly longer than 3.1 months in the ipilimumab group. Objective response rates were 49% in TIL group and 21% (95% CI, 13 to 32) of patients in ipilimumab group. 20% of patients in the TIL group achieved complete response, compared to 7% in the ipilimumab group. At the time of the data cutoff, the overall median follow-up was 33.0 months. Median overall survival was 25.8 months in the TIL group and 18.9 months in the ipilimumab group. In regards to safety, treatment related adverse events occurred in all patients in the TIL group and in 96% of those in the ipilimumab group. All patients in the TIL group experienced grade 3 adverse events, largely due to chemotherapy-related myelosuppression, while 57% of patients in the ipilimumab group experienced grade 3 or higher adverse events. Overall, patients in the TIL group had higher mean health-related quality of life scores than those in the ipilimumab group, though scores varied for different symptoms.
Discussion Points
Improving outcomes for patients with ICI-refractory disease is a critical need. The treatment landscape is evolving and clinicians currently only have a few options to choose from when patients progress on first-line anti-PD-1 monotherapy. Commercially-available options include monotherapy ipilimumab, dual ICB with ipilimumab/nivolumab or ipilimumab/relatlimab and combination targeted therapies for patients with actionable BRAF V600 mutations. When provided with a clinical vignette of ICI-refractory wild-type melanoma, the vast majority of the diverse group of CCO attendees at the January 9th journal club that treat melanoma (Figure 5) responded that they would have selected combination ipilimumab/nivolumab (Figure 6). Given the patient’s ECOG status of 2, one respondent replied that they have recommended combination CTLA-4/LAG-3 in this case. None of the practioners would have recommended monotherapy ipilimumab.
Attendees recognized that while dual ICB and combination oncogene-targeted therapies can produce responses in the second-line and beyond, meaningful clinical benefit is limited to a fraction of patients. For many years, TIL therapy has produced intriguing results in small, non-comparative investigations. Given the lack of commercial approval, TIL therapy has not been widely adopted. However, the majority of melanoma clinicians at the January 9th CCO JC have recommended TIL therapy previously as part of their practice (Figure 7). Some participants also recognized the importance of this paper as it is one of very few prospective, randomized trials of TIL therapy. While this is, in part, due to the challenges in identifying an appropriate control group, the data reported did identify a significant improvement in cinical outcome and a high ORR of 49% in a refractory melanoma population.
Furthermore, most were affiliates at institutions with a cell therapy group capable of delivery TIL therapy (Figure 8).
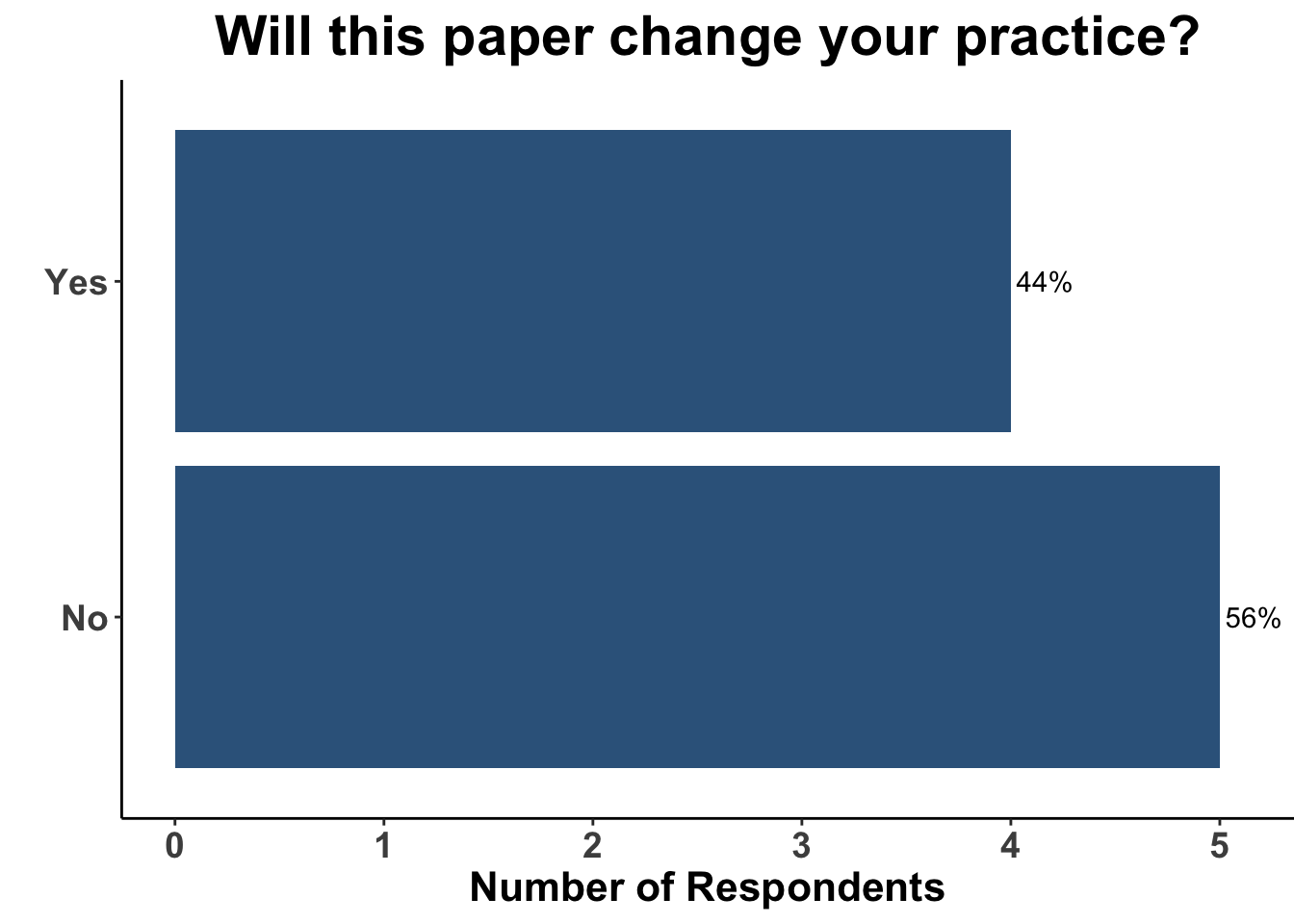
Thus, the results from Rohaan et al. were of a considerable interest. TIL therapy was associated with a clinically meaningful 50% reduction in the hazard for progression or death compared with monotherapy ipilimumab. Despite the majority of patients being treated previously with immunotherapy, nearly one-half of the patients experienced a response to TIL therapy. Importantly, responses were durable in the majority of patients that experienced an initial response. These data corroborate the findings in C-144-01, in which 15-20% of the heavily pre-treated cohort experienced durable responses following administration of lifileucel (Sarnaik et al. 2021). Taken collectively, TIL therapy is likely to become a standard option in the field. Indeed, Iovance, the sponsor of C-144-01, is expecting the rolling Biologics Licences Application (BLA) submission of lifileucel to be completed in the first half of 2023. Interestingly, while the attendees verbalized that the study by Rohaan et al. was a landmark investigation, less than half replied that it would change their practice (Figure 9).
Indeed, when presented with the same case of refractory advanced melanoma, but given the option of having TIL therapy available, only 1/9 clinicians responded that they would recommend TIL therapy (Figure 10).
Only a minority of respondents replied that if approved, TIL therapy would become their preferred treatment strategy for PD-1 refractory melanoma (Figure 11). Toxicity and logistics of use were both discussed as considerations when selecting patients that might benefit from TIL therapy.
Despite the promise of TIL therapy, there are several challenges with its adoption and implementation. Most notably are the risks associate with its use. Grade 3 or higher treatment related adverse event (TRAEs) were nearly universal in both C-144-01 and the study by Rohaan et al. Granted, the vast majority were expected from the lymphodepleting agents used as part of the conditioning regimen, were transient and manageable. Nevertheless, 86% experienced the potentially life-threatening complication of febrile neutropenia and 10% of patients required transfer to the intensive care unit. When compared to the other treatment options for refractory disease, TIL therapy appears to offer a trade off of enhanced efficacy at the expense of increased toxicity (Figure 12).
Another consideration and potential barrier to TIL therapy are the logistics required for preparation. Adequate and accessible tumor tissue is needed, as is several weeks for cultivation and expansion of donor cells. Therefore, TILs may be less appropirate for patients with hard-to-access metastases and/or rapidly progressing disease. Thus, the use of TIL therapy is likely to be individualized and utilized in centers with experienced practioners. Nevertheless, the studies by Sarnaik et al. and Rohaan et al. reported a higher rate of TIL generation after harvesting suggesting improved technical capabilities to generate TIL are now available. In summary, these reports represent important contributions to the field and will likely lead to clinicians having additional options for their patients in the near future.
Materials and Methods
This Perspectives on the Science piece was published using Quarto®. The survey was conducted using REDCap® (Harris et al. 2009). The figures depicting the survey data were created using R (version 4.0.0) and the tidyverse suite of packages (Wickham et al. 2019), including ggplot2 (Wickham 2016). The figure depicting FDA approved therapies in melanoma was created using the skincancerRx package (Miller and Shalhout 2022).
Bibliography
Appendix
Citation
@article{kim2023,
author = {Kim, Emily Y. and Shalhout, Sophia Z. and Kaufman, Howard L.
and Emerick, Kevin S. and Patel, Vishal A. and Garmen, Khalid and
Brownell, Isaac and Miller, David M.},
publisher = {Society of Cutaneous Oncology},
title = {Tumor-Infiltrating {Lymphocyte} {Therapy} for {Advanced}
{Melanoma:} {Ready} for {Prime} {Time?}},
journal = {Journal of Cutaneous Oncology},
volume = {1},
number = {1},
date = {2023-01-09},
url = {https://journalofcutaneousoncology.io/perspectives/Vol_1_Issue_1/til_therapy_for_advanced_melanoma/},
doi = {10.59449/joco.2023.01.09},
issn = {2837-1933},
langid = {en}
}