A Closer Look: Evaluating Mohs Surgery’s Role in the Treatment of Invasive Melanoma of the Head and Neck
Mohs micrographic surgery, melanoma
Featured Article
Brandon T. Beal, Jeremy Udkoff, Leora Aizman, Jeremy Etzkorn, John A. Zitelli, Christopher J. Miller, Thuzar M. Shin, Joseph F. Sobanko, David G. Brodland, Outcomes of invasive melanoma of the head and neck treated with Mohs micrographic surgery – A multicenter study, Journal of the American Academy of Dermatology, Volume 89, Issue 3, 2023, Pages 544-550, ISSN 0190-9622, https://doi.org/10.1016/j.jaad.2022.12.038.
Introduction
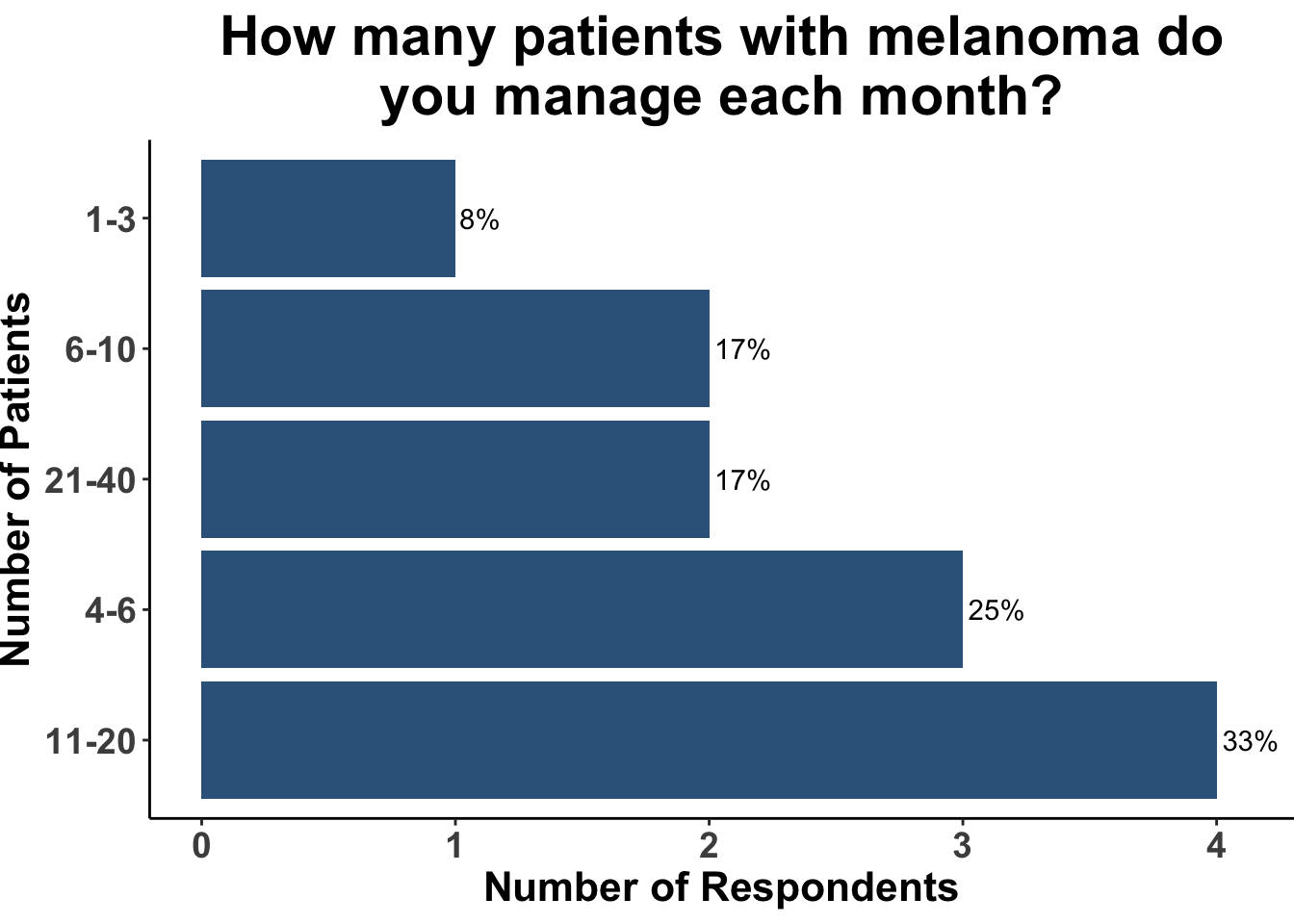
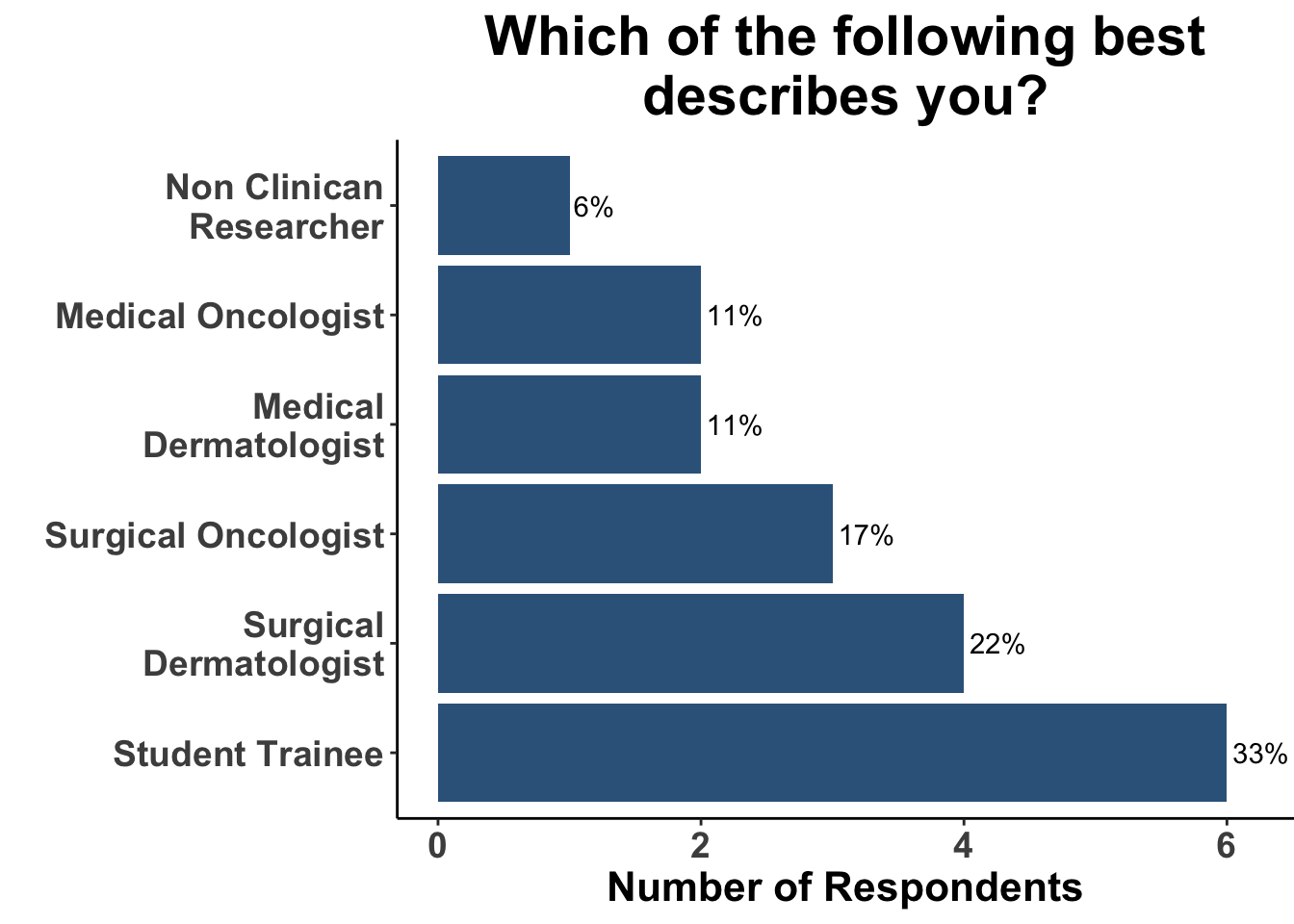
On November 6th, 2023 the multi-institutional Society of Cutaneous Oncology (SoCO) Journal Club (JC) reviewed the Journal of American Academy of Dermatology article “Outcomes of invasive melanoma of the head and neck treated with Mohs micrographic surgery – A multicenter study”1. Participants included clinicians and investigators from Massachusetts General Hospital, Massachusetts Eye and Ear, the National Institutes of Health, the Dana-Farber Cancer Institute, George Washington University School of Medicine, Georgetown University School of Medicine and the University of Pittsburgh (Figure 1), with a range of experience managing melanoma (Figure 2). This Perspectives on the Science article reflects the authors’ views after the Journal Club. Please note that it does not represent the views of other SoCO members or affiliated institutions. In this article, we provide a summary of the article as well as discuss its strengths and limitations.
Background for the Study
The management of invasive melanoma on the head and neck presents significant challenges. These challenges include unique surgical locations, positive excision margins, local recurrence, a roughly 10% risk each for upstaging and a tenfold increased likelihood of a flap or graft reconstruction, often referred to as the rule of 10s.2
Presently, the National Comprehensive Cancer Network (NCCN) guidelines for invasive melanoma stem from findings across six randomized control trials (RCTs) involving a total of 4,206 cases of melanoma with a mere 27 (0.6%) of these of these being located on the head or neck (Table 1).3–9 Thus, the current body of consistent clinical data guiding the selection of surgical management or margins for invasive melanoma in the head and neck (H&N) region is limited.
The traditional approach of treating H&N melanomas using conventional excision (CE) can be challenging because the anatomic sites may not easily accommodate wide excisions margins of 1-2 cm. In addition, there is data to suggest that CE may result in increased rates of positive margins, local recurrences, and disease specific deaths compared to CE for invasive trunk and extremity melanoma. Mohs micrographic surgery (MMS), a technique long used in the management of non-melanoma skin cancer, has increasingly been incorporated in the treatment of in situ, as well as invasive, melanoma (Tables 2-3).1,10–36 Some reports have demonstrated that MMS can be a more comprehensive method for assessing peripheral and deep surgical margins during melanoma treatment.34,37,38 A retrospective study involving 36 cases of melanoma in situ treated with the method of serial cross-sectioning used in these standard evaluations at intervals of 1, 2, 4, and 10 mm could only detect positive margins 58%, 37%, 19%, and 7% of the time, respectively.39 Such data provide rationale for adopting complete histological margin control to reduce the risk of recurrence significantly. Staged excision allows for comprehensive histological margin control, aiming to reduce the risk of recurrence more effectively. One of the limitations of staged excisions is that procedures may extend over days to weeks.
In contrast, MMS may offer complete margin assessment during melanoma treatment without delay in subsequent reconstruction.34,37,39–41 The initial use of MMS – one form of complete margin control – faced challenges given concern, by some, in accurately identifying melanocytes in frozen sections. However, the utilization of immunohistochemical (IHC) stains, especially Melanoma Antigen Recognized by T cells (MART-1), to aid in frozen section analysis has made the identification of melanoma reliably possible.37,38 Frozen sections using MART-1 have been shown to provide equivalent detection of melanoma as MART-1 stained permanent sections.42 A recent survey showed that 91% to 97.8% of Mohs surgeons trained in the last two decades are now using MART-1 as their exclusive choice for IHC staining during MMS on melanoma.37
These findings highlight the potential value of integrating MMS and IHC techniques in the treatment of melanoma, particularly in the head and neck region.
Study Design
This retrospective study, conducted at Zitelli & Brodland, P.C. and the University of Pennsylvania across 12 years, reviewed 785 cases of invasive melanomas in the head and neck region, treated with MMS using MART-1 immunohistochemical stains. Eligible participants were adults with biopsy-confirmed primary invasive melanoma on the head and neck, absent of metastatic disease on presentation. Excluded from the study were melanoma in situ, tumors on the trunk or extremities, previously treated cases, and participants presenting with nodal or distant metastasis.
Study Results
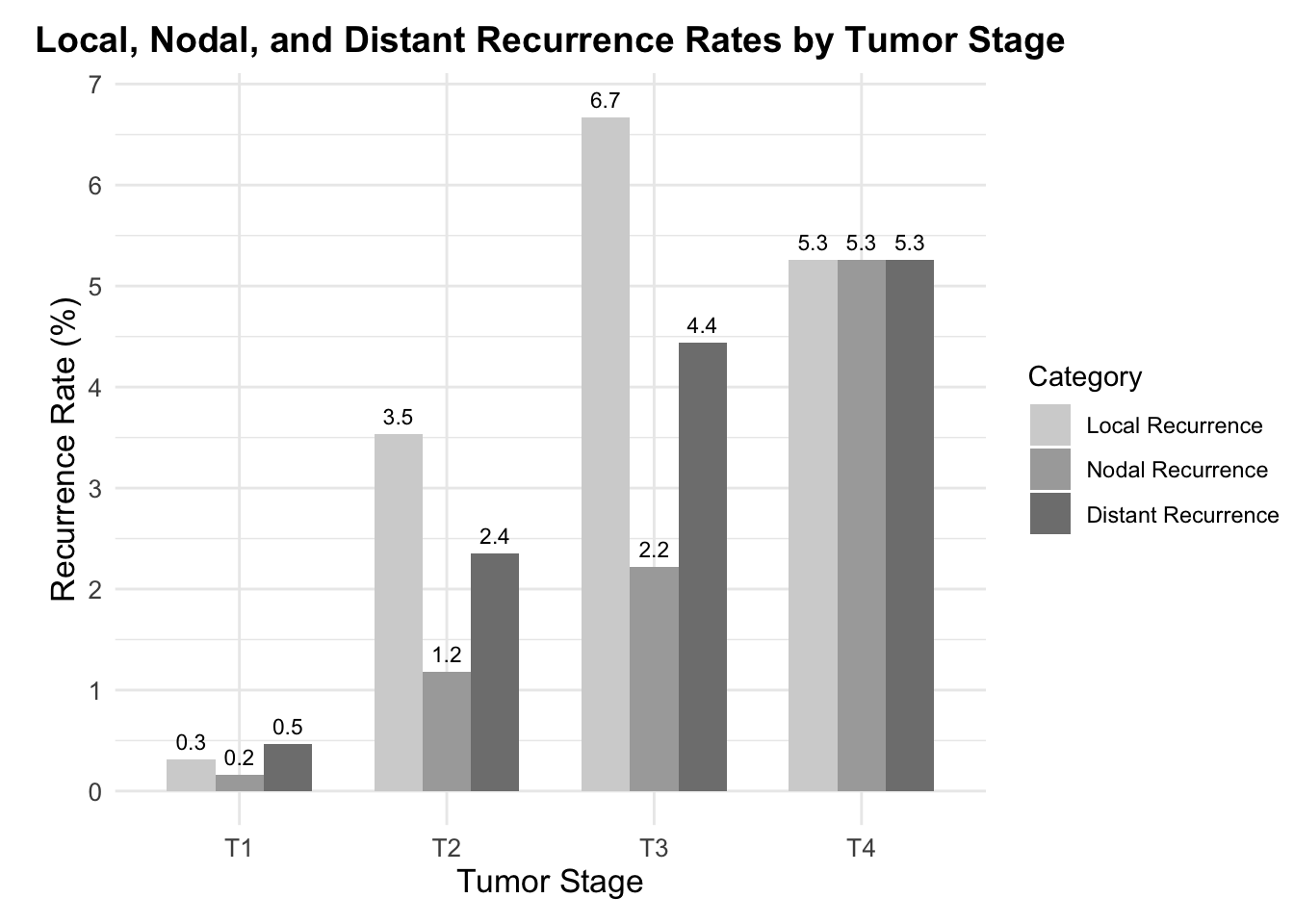
The study encompassed the treatment of 785 melanoma cases with variances in thickness between 0.3mm and 8.5mm through MMS. Following treatment, recurrence details were analyzed which revealed that local recurrence was observed in 0.51% (approximately 4 out of 785 cases), nodal recurrence was seen in 1.0% (approximately 8 out of 785 cases), and distant recurrence in 1.1% (approximately 9 out of 785 cases). When delving into the recurrence rates according to tumor stages T1 to T4, the study indicated that local recurrence stood at 0.16% for T1 stage (1 out of 636 cases), 1.18% for T2 stage (1 out of 85 cases), 2.22% for T3 stage (1 out of 45 cases), and 5.26% for T4 stage (1 out of 19 cases) (Figure 3). The survival rates after five and ten years were also promising, with disease-specific survival calculated at 96.8% (with a 95% confidence interval between 95.0% and 98.5%) and 93.4% (with a 95% confidence interval between 88.5% and 98.3%) respectively.
Discussion
The potential for same-day complete surgical margin analysis in head and neck invasive melanomas presents potential benefits compared to the conventional excisional method. These advantages include (1) a more complete resection of malignant cells due to more thorough margin assessment; and (2) achieving negative margins prior to reconstruction without delay. The use of a staged excision protocol may indeed achieve the former, but not necessarily the latter. Whether the application of MMS provides equal or superior results to conventional methods is yet to be elucidated with prospective data.
Beal et al. 2023 provides retrospective data from two institutions exploring the use of MMS for invasive melanoma. Strengths of this study include a sizable cohort of 785 cases treated with MMS for invasive melanomas on the head and neck over a 12-year period. Two distinct clinical sites using similar surgical techniques were included without discernible differences in terms of local recurrence (LR), nodal recurrence (NR), and distal recurrence (DR). This suggests that the results are not specific to a single practice. Given that the majority of patients (81%) had a Breslow depth of 1 mm or less, the low overall LR, NR, and DR noted in the study for invasive melanoma may be most confidently inferred for early invasive disease.
The limitations of the study include a variable follow-up method, with some individuals followed up through phone calls while others were considered based on their last physical examination recorded in their medical records. The study also lacked a matched control group for a clear comparison, or even a historical control population. There was a shortage of detailed reporting on certain clinical characteristics such as the number of surgical stages required for the complete clearance of the tumor.
Patients in the study eligible for sentinel lymph node biopsies (SLNB) based on the NCCN guidelines were offered testing; however, this data was not tracked, which would have been helpful in understanding the reported recurrence rates. Also not included was the technical challenges of performing SLNB in conjunction with MMS; understanding how this was or was not incorporated would also be helpful in understanding the generalizability of this approach. SLNB data has demonstrated relevance in providing an accurate prognostication as well as potential improvements in disease management in the context of a shifting therapeutic landscape, which might influence recurrence rates.41,43 The study included patients up until 2017 during a time when the management of metastatic melanoma was advancing due to the emergence of approval for immune checkpoint inhibitors (ICI) and BRAF and MEK inhibitors. The U.S FDA, in 2011, approved the use of the anti–CTLA-4 antibody ipilimumab for patients with stage IV melanoma, and later in 2014 approved anti–PD-1 antibodies nivolumab and pembrolizumab for second line treatment. These therapies have shifted the survival rate from less than 10% to 30% to 50% over the past decade.40,44 Additionally, in 2015, the NCCN recommended, and the FDA approved the use of PD-1 ICI as the first line therapy for patients with stage IV melanoma. More recently, immunotherapy and targeted therapy has also been approved for adjuvant therapy, including patients with Stage IIB-IV melanoma. Understanding the influence of these novel therapies on patient survival rates necessitates further research.
Another limitation is that the study did not directly compare MMS to CE or staged excision. A comparative analysis would have been useful to assess the relative effectiveness of the two strategies and would have reinforced their support of MMS for invasive melanoma on the head and neck rather than comparing against historical data. The retrospective, non-randomized design of the study was also a subject of discussion, leading to an agreement on the need for future prospective studies.
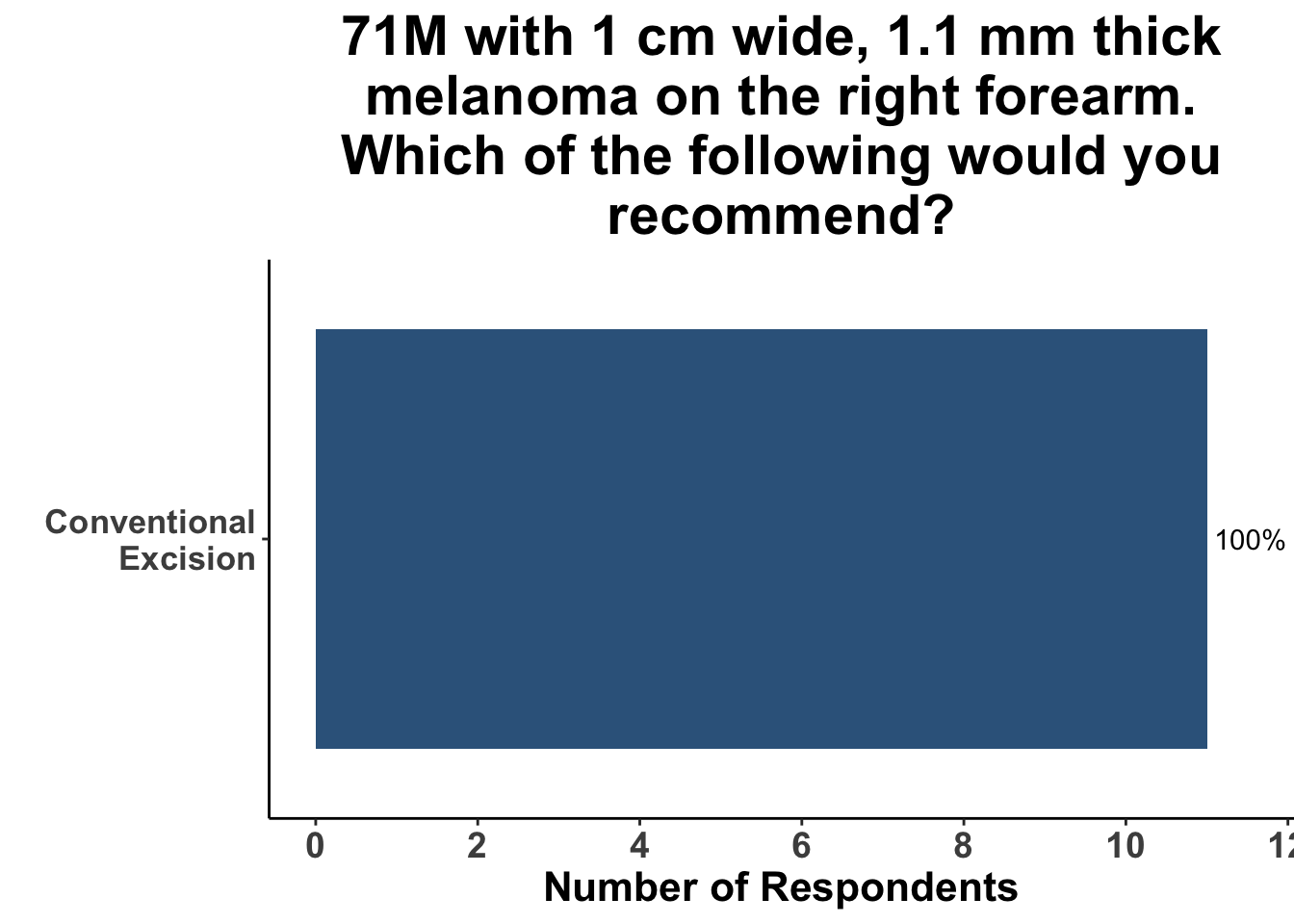
Inputs were sought from the experienced, multi-disciplinary SoCO group regarding their preferred treatment modality based on hypothetical clinical scenarios. In consideration of a case involving a 71-year-old male patient with a 1 cm wide, 1.1 mm thick melanoma on the vertex scalp, a quarter of the participants expressed a preference for MMS as their treatment method while the remaining 75% supported the conventional excision technique. Another case, involving the same measurements of melanoma but located on the right forearm of the male patient, saw unanimous consensus for conventional excision as the treatment method (Figures 4 and 5).
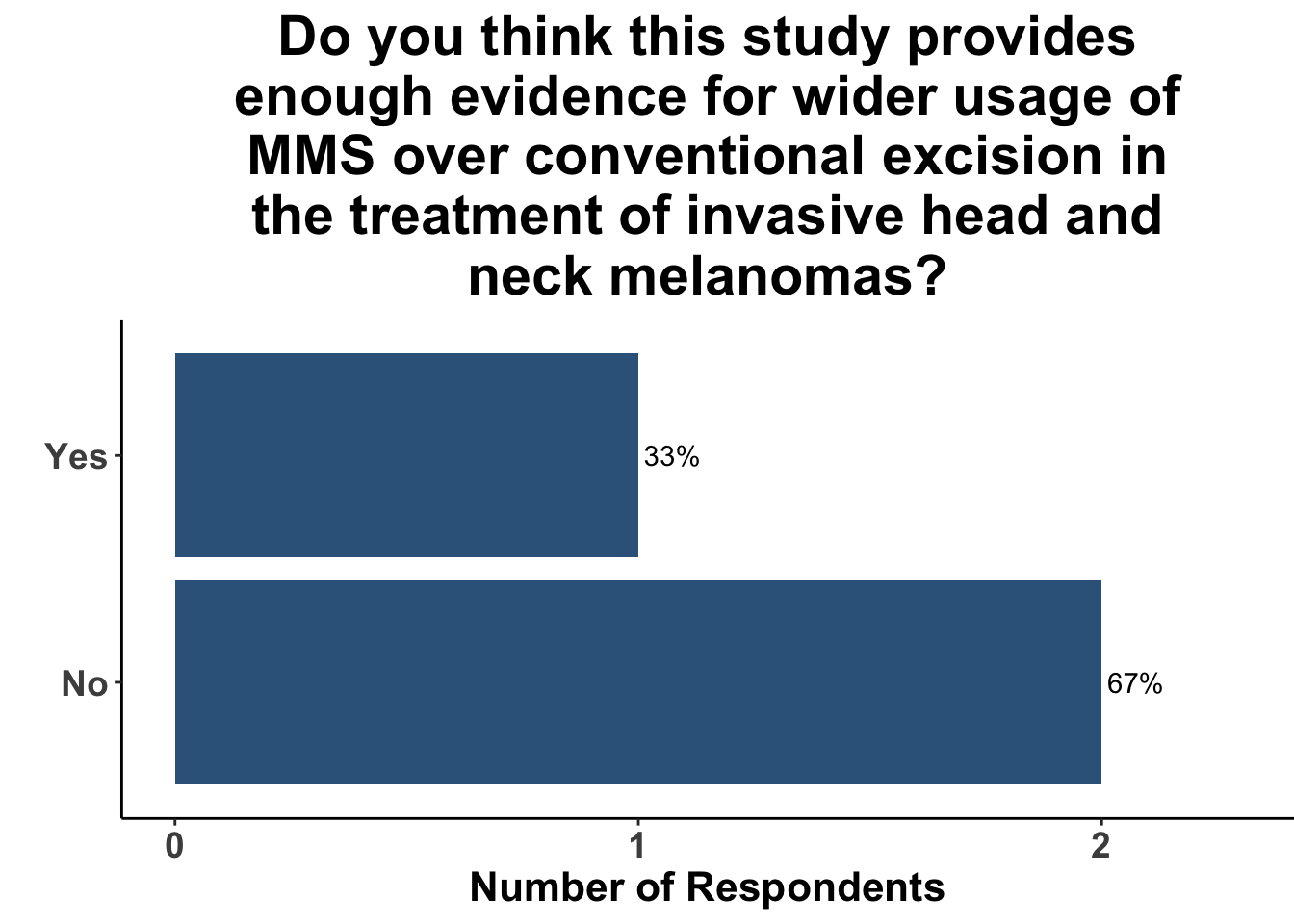
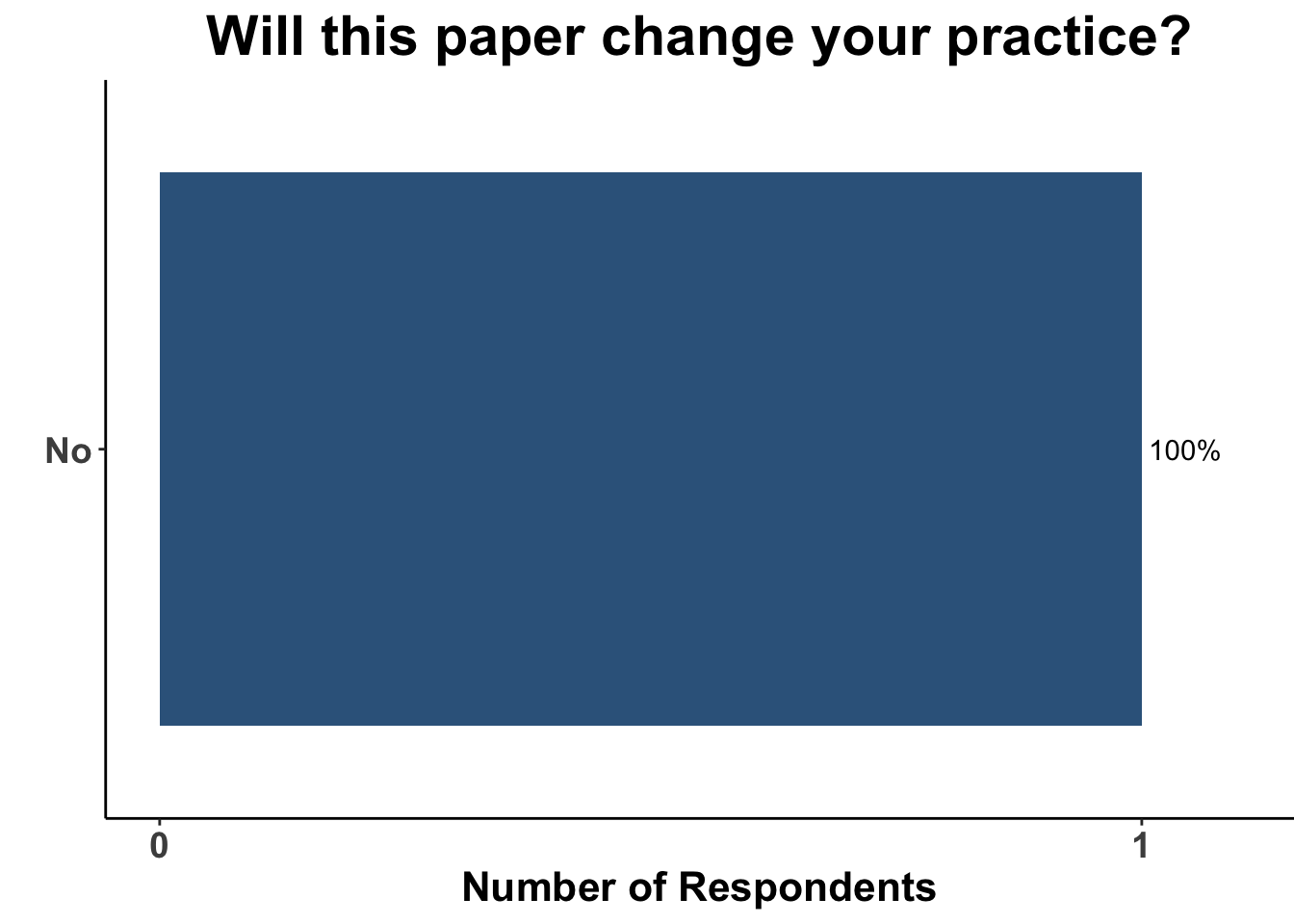
After discussion of the article, 17% of respondents felt the study provided adequate evidence for a shift towards MMS for the treatment of invasive head and neck melanomas (Figure 6). In terms of the utility of MMS over conventional excision, one-fifth of the clinicians anticipated potential changes in their medical practice (Figure 7). A majority of the participants (91%) felt that while MMS for invasive head and neck melanoma may show promise, challenges remain in terms of incorporating MMS into their own practice (Figure 8). This suggests the opportunity for prospective randomized trials or even matched case-control studies comparing surgical interventions.
Given that the SoCO group practitioners form a diverse group with various experiences managing melanoma, the survey data collected gives insight into the acceptance and potential deterrents to integrating MMS into mainstream practice.
Materials and Methods
This Perspectives on the Science piece was published using Quarto®. The survey was conducted using REDCap®.45 The figures depicting the survey data were created using R (version 4.0.0) and the tidyverse suite of packages,46 including ggplot2.47 The image on the “Perspectives on the Science” page was created by the authors (DMM) using the rosemary package.48
Bibliography
Appendix
Citation
@article{ching2024,
author = {Ching, Lauren and Strong, Jennnifer and Lee, Truelian and
Kaufman, Howard L. and Emerick, Kevin S. and Kim, Emily Y. and
Patel, Vishal A. and Brownell, Isaac and Singh, Kritika and Neel,
Victor A. and Miller, David M. and Gupta, Sameer},
publisher = {Society of Cutaneous Oncology},
title = {A {Closer} {Look:} {Evaluating} {Mohs} {Surgery’s} {Role} in
the {Treatment} of {Invasive} {Melanoma} of the {Head} and {Neck}},
journal = {Journal of Cutaneous Oncology},
volume = {2},
number = {1},
date = {2024-01-24},
url = {https://journalofcutaneousoncology.io/perspectives/Vol_2_Issue_1/mohs_for_melanoma/},
doi = {10.59449/joco.2024.01.24},
issn = {2837-1933},
langid = {en}
}